

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50440868 CHEMBL2431617
SMILES: CC[C@H](C)[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)c1ccno1)C(=O)N1CCC2(CC1)C=Cc1ccccc21
InChI Key: InChIKey=DCUDDCGUKZLQLN-MCOVPRHSSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868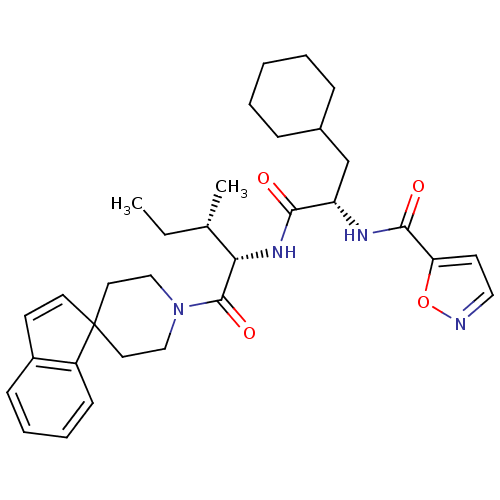 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University Curated by ChEMBL | Assay Description Antagonist activity at protease-activated receptor 2 (unknown origin) | Bioorg Med Chem 23: 7717-27 (2015) BindingDB Entry DOI: 10.7270/Q2VH5QP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of trypsin-induced ca2+ release preincubated for 15 mins measured after 1 hr ... | Bioorg Med Chem Lett 26: 986-91 (2016) BindingDB Entry DOI: 10.7270/Q2PG1TKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of trypsin-induced ca2+ release preincubated for 15 mins measured after 1 hr ... | Bioorg Med Chem Lett 26: 986-91 (2016) BindingDB Entry DOI: 10.7270/Q2PG1TKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of 2f-LIGRLO-NH2-induced ca2+ release preincubated for 15 mins measured after... | Bioorg Med Chem Lett 26: 986-91 (2016) BindingDB Entry DOI: 10.7270/Q2PG1TKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of agonist-induced calcium mobilization | J Med Chem 56: 7477-97 (2013) Article DOI: 10.1021/jm400638v BindingDB Entry DOI: 10.7270/Q2NC62MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of 2f-LIGRLO-NH2-stimulated intracellular calcium release preincubated for 30... | ACS Med Chem Lett 7: 1179-1184 (2016) Article DOI: 10.1021/acsmedchemlett.6b00306 BindingDB Entry DOI: 10.7270/Q2PN97MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of endogenous trypsin-stimulated intracellular calcium release by Fluo-3 AM d... | ACS Med Chem Lett 7: 1179-1184 (2016) Article DOI: 10.1021/acsmedchemlett.6b00306 BindingDB Entry DOI: 10.7270/Q2PN97MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of 2f-LIGRLO-NH2-stimulated intracellular calcium release preincubated for 30... | ACS Med Chem Lett 7: 1179-1184 (2016) Article DOI: 10.1021/acsmedchemlett.6b00306 BindingDB Entry DOI: 10.7270/Q2PN97MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50440868 (CHEMBL2431617) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human HT-29 cells assessed as inhibition of 2f-LIGRLO-NH2-induced ca2+ release preincubated for 15 mins measured after... | Bioorg Med Chem Lett 26: 986-91 (2016) BindingDB Entry DOI: 10.7270/Q2PG1TKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||