

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50445060 CHEMBL3098773
SMILES: C[C@@H]1CC\C(=C/C(/C)=C\C=C\C(\C)=C\C(O)=O)c2ccccc12
InChI Key: InChIKey=IUAWGSHMQSZQSY-ARGJGUSHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NR2B Channel Blocker (RAT) | BDBM50445060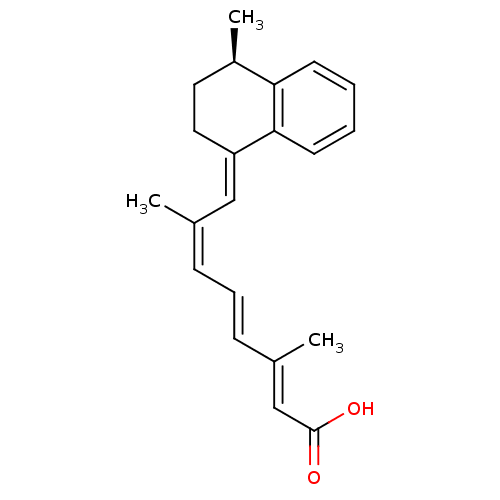 (CHEMBL3098773) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Agonist activity at RXR-alpha in rat R3KE cells infected with oncogene KLF4-ER assessed as inhibition of KLF4-mediated oncogenic transformation | J Med Chem 57: 5370-80 (2014) Article DOI: 10.1021/jm5004792 BindingDB Entry DOI: 10.7270/Q2183821 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NR2B Channel Blocker (RAT) | BDBM50445060 (CHEMBL3098773) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Agonist activity at RXRalpha in rat RK3E cells assessed as transcriptional activation by luciferase reporter gene assay | Bioorg Med Chem 22: 178-85 (2013) Article DOI: 10.1016/j.bmc.2013.11.039 BindingDB Entry DOI: 10.7270/Q2MW2JMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50445060 (CHEMBL3098773) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Agonist activity at Gal4-fused human RXR-alpha expressed in HEK293 cells assessed as receptor-mediated transcriptional activity treated 24 hrs after ... | J Med Chem 57: 5370-80 (2014) Article DOI: 10.1021/jm5004792 BindingDB Entry DOI: 10.7270/Q2183821 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50445060 (CHEMBL3098773) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Binding affinity to human RXR-alpha-ligand binding domain homodimers by fluorescence quenching method | J Med Chem 57: 5370-80 (2014) Article DOI: 10.1021/jm5004792 BindingDB Entry DOI: 10.7270/Q2183821 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||