

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50451423 CHEMBL4217392
SMILES: CS(=O)(=O)N1CCN(Cc2nc3c(nc(cn3n2)-c2cnc(N)nc2)N2CCOCC2)CC1
InChI Key: InChIKey=LFNUJWZBIXHQJT-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p110α/p85α (Homo sapiens (Human)) | BDBM50451423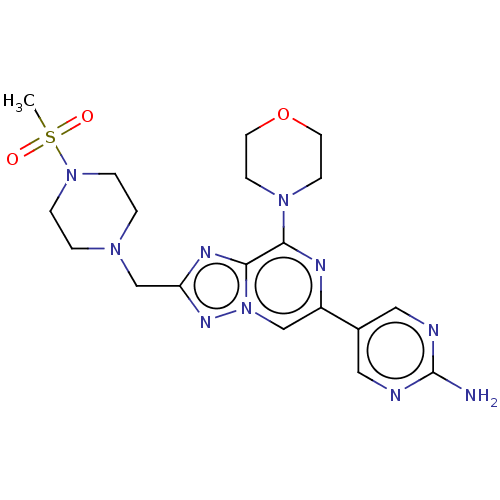 (CHEMBL4217392) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO) Curated by ChEMBL | Assay Description Inhibition of full-length human P110alpha (1 to 1068 residues)/N-terminal GST-fused p85alpha (1 to 724 residues) expressed in baculovirus expression ... | Bioorg Med Chem Lett 27: 4794-4799 (2017) Article DOI: 10.1016/j.bmcl.2017.09.059 BindingDB Entry DOI: 10.7270/Q2F76G4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoinositide 3-Kinase (PI3K), delta (Homo sapiens (Human)) | BDBM50451423 (CHEMBL4217392) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO) Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal His6-tagged p110delta/human full-length p85alpha expressed in baculovirus infected Sf21 insect... | Bioorg Med Chem Lett 27: 4794-4799 (2017) Article DOI: 10.1016/j.bmcl.2017.09.059 BindingDB Entry DOI: 10.7270/Q2F76G4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50451423 (CHEMBL4217392) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO) Curated by ChEMBL | Assay Description Inhibition of recombinant full-length human N-terminal His6-tagged p110gamma expressed in baculovirus infected Sf21 insect cells using PIP2 as substr... | Bioorg Med Chem Lett 27: 4794-4799 (2017) Article DOI: 10.1016/j.bmcl.2017.09.059 BindingDB Entry DOI: 10.7270/Q2F76G4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p110β/p85α (Homo sapiens (Human)) | BDBM50451423 (CHEMBL4217392) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO) Curated by ChEMBL | Assay Description Inhibition of p110beta/85alpha (unknown origin) using PIP2 as substrate by HTRF assay | Bioorg Med Chem Lett 27: 4794-4799 (2017) Article DOI: 10.1016/j.bmcl.2017.09.059 BindingDB Entry DOI: 10.7270/Q2F76G4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50451423 (CHEMBL4217392) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO) Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system by AlexaFluor647-... | Bioorg Med Chem Lett 27: 4794-4799 (2017) Article DOI: 10.1016/j.bmcl.2017.09.059 BindingDB Entry DOI: 10.7270/Q2F76G4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50451423 (CHEMBL4217392) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO) Curated by ChEMBL | Assay Description Inhibition of human ERG by Tracer Red dye based fluorescence polarization assay | Bioorg Med Chem Lett 27: 4794-4799 (2017) Article DOI: 10.1016/j.bmcl.2017.09.059 BindingDB Entry DOI: 10.7270/Q2F76G4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||