

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50455103 CHEMBL4202850
SMILES: CN1CCCC[C@H](NC(=O)c2cc(C)on2)C(=O)N2C[C@@H](C[C@H]2C(=O)N[C@@H](CCC1=O)C=O)c1ccccc1
InChI Key: InChIKey=GWMCHRBWEZGESK-VCHRRKICSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus 14) | BDBM50455103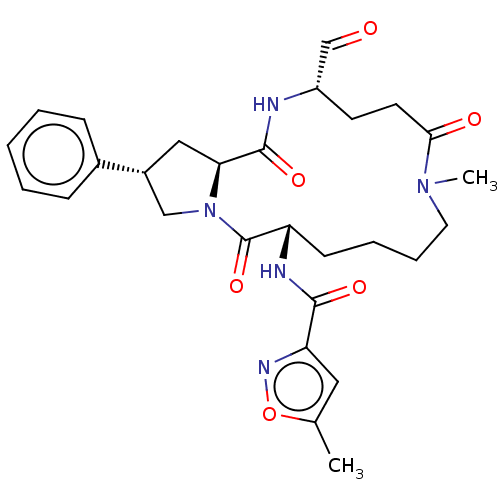 (CHEMBL4202850) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||