

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50458749 CHEMBL4211915
SMILES: C[C@H]1COCCN1c1nc(cc(n1)C1(CC1)S(C)(=O)=O)-c1cnc(N)cc1F
InChI Key: InChIKey=LBIPJQHFIZPQHA-NSHDSACASA-N
Data: 17 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p110α/p85α (Homo sapiens (Human)) | BDBM50458749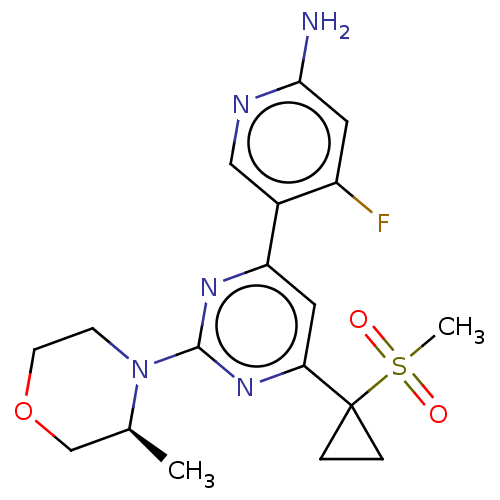 (CHEMBL4211915) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length N-terminal His-tagged p110alpha/p85alpha expressed in baculovirus expression system using PIP2 as substra... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50458749 (CHEMBL4211915) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human ERG expressed in CHO cells by automated whole cell patch clamp Qpatch method | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoinositide 3-Kinase (PI3K), delta (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal His6-tagged p110delta/recombinant human full length p85alpha expressed in baculovirus infected... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4EBP1 a... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p110α/p85α (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged p85alpha/p110alpha E542K mutant expressed in insect cells | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate pretreated for 5 mins followed by NADPH addition and measured after 10 m... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate pretreated for 5 mins followed by NADPH addition and measured after 10 mi... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate pretreated for 5 mins followed by NADPH addition and measured after 10 m... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-Mephenytoin as substrate pretreated for 5 mins followed by NADPH addition and measured after ... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate pretreated for 5 mins followed by NADPH addition and measured after 10 mi... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p110α/p85α (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged p85alpha/p110alpha E545K mutant expressed in insect cells | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate pretreated for 5 mins followed by NADPH addition and measured after 5 min... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of p110alpha H1047R mutant (unknown origin) | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| p110β/p85α (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal His6-tagged p110beta/recombinant human full length p85alpha expressed in baculovirus infected ... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged p110gamma expressed in baculovirus expression system using PIP2 as substrate measured after 1 ... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate pretreated for 5 mins followed by NADPH addition and measured after 10 m... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50458749 (CHEMBL4211915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Haiyan Pharmaceutical Technology Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate pretreated for 5 mins followed by NADPH addition and measured after 5 ... | ACS Med Chem Lett 9: 719-724 (2018) Article DOI: 10.1021/acsmedchemlett.8b00167 BindingDB Entry DOI: 10.7270/Q21V5HK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||