

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50459052 CHEMBL4211642
SMILES: COc1ccc(c(OC)n1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1
InChI Key: InChIKey=ZVOOPGACTWEGSR-UHFFFAOYSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator/surface receptor (Homo sapiens (Human)) | BDBM50459052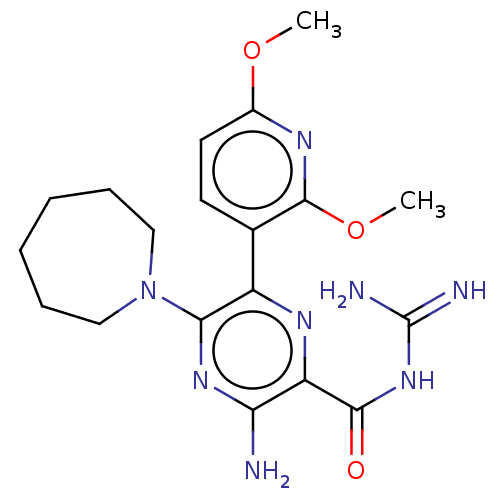 (CHEMBL4211642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 884 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay | J Med Chem 61: 8299-8320 (2018) Article DOI: 10.1021/acs.jmedchem.8b00838 BindingDB Entry DOI: 10.7270/Q2QF8WH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||