

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@@]1(N=C(N)O[C@H](CF)[C@@H]1F)c1cc(NC(=O)c2cnc(cn2)C(F)F)ccc1F
InChI Key: InChIKey=ASUXJKFZYWODQA-QWQRMKEZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50462006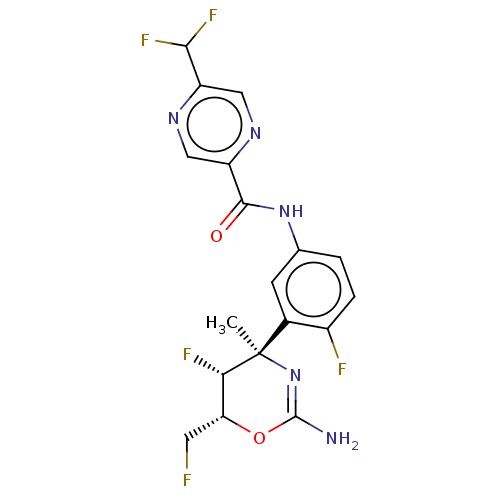 (CHEMBL4226730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 15 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 5525-5546 (2018) Article DOI: 10.1021/acs.jmedchem.8b00011 BindingDB Entry DOI: 10.7270/Q2SB48DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50462006 (CHEMBL4226730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells harboring wild-type human beta-APP assessed as reduction in secreted amyloid beta (1 to 40) after 24 hrs b... | J Med Chem 61: 5525-5546 (2018) Article DOI: 10.1021/acs.jmedchem.8b00011 BindingDB Entry DOI: 10.7270/Q2SB48DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50462006 (CHEMBL4226730) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (43 to 454 residues) expressed in Escherichia coli BL21(DE3) using Biotin-epsilon-amino-n-capronic acid-SEVNLDA... | J Med Chem 61: 5525-5546 (2018) Article DOI: 10.1021/acs.jmedchem.8b00011 BindingDB Entry DOI: 10.7270/Q2SB48DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||