

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50464867 CHEMBL4289783
SMILES: Fc1ccc(N2CCN(CCCc3c[nH]c4ccc(F)cc34)CC2)c(c1)-c1ccccc1
InChI Key: InChIKey=GPVZPQVIHIFYGO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50464867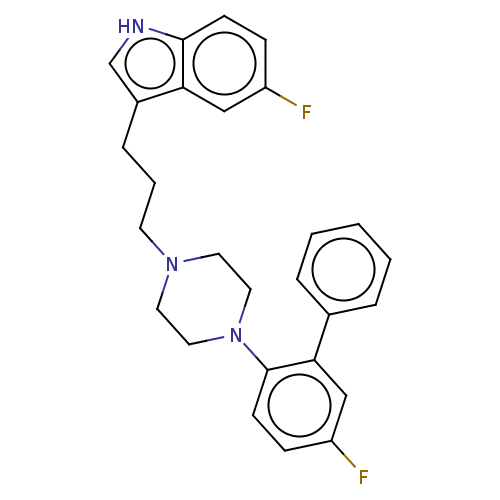 (CHEMBL4289783) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]LSD from recombinant human 5-HT7 receptor expressed in CHO cell membranes after 120 mins by TopCount scintillation counting metho... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50464867 (CHEMBL4289783) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1-adrenoceptor (unknown origin) in cerebral cortex membranes after 60 mins by TopCount scintillation counting ... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50464867 (CHEMBL4289783) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from recombinant human 5-HT1A receptor expressed in HEK293 cell membranes after 60 mins by TopCount scintillation count... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50464867 (CHEMBL4289783) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO-K1 cells assessed as reduction in tail current amplitude at -80 mV holding potential after 2 mins by path cl... | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50464867 (CHEMBL4289783) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Inhibition of [3H]serotonin reuptake in rat brain synaptosomes SERT after 15 mins by TopCount scintillation counting method | Eur J Med Chem 144: 701-715 (2018) Article DOI: 10.1016/j.ejmech.2017.12.063 BindingDB Entry DOI: 10.7270/Q26D5WP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||