

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50465712 CHEMBL4284414
SMILES: CCOC(=O)c1ccc(CN(Cc2ccc(cc2)-c2cccc(c2)S(C)(=O)=O)S(=O)(=O)c2c(C)cc(C)cc2C)o1
InChI Key: InChIKey=HDZWHJYZJWLTAG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50465712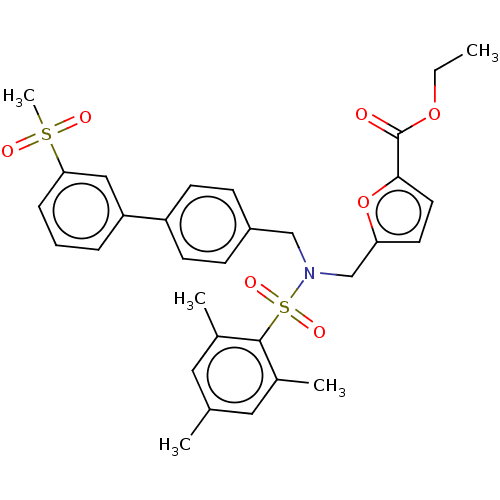 (CHEMBL4284414) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 352 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... | Eur J Med Chem 178: 458-467 (2019) Article DOI: 10.1016/j.ejmech.2019.06.011 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50465712 (CHEMBL4284414) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Inverse agonist activity at LXRbeta (unknown origin) expressed in HEK293 cells 24 hrs by Dual-Glo luciferase assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50465712 (CHEMBL4284414) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50465712 (CHEMBL4284414) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50465712 (CHEMBL4284414) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Inverse agonist activity at LXRalpha (unknown origin) expressed in HEK293 cells 24 hrs by Dual-Glo luciferase assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||