

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@@]12NC(=O)[C@H](NC(=O)[C@@H](CC3C(=O)Nc4cc(ccc34)-c3cc1cc(Oc1ccc(C[C@H](N(C)C(=O)[C@H](NC2=O)c2cc(Cl)c(O)c(Cl)c2)C(=O)N[C@@H](C(O)=O)c2ccc(O)cc2)cc1)c3O)NC(=O)C(=O)c1cc(Cl)c(O)c(Cl)c1)c1cc(Cl)c(O)c(Cl)c1
InChI Key: InChIKey=ZFUGTFLRENMBAG-HYTWXIRXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM50478735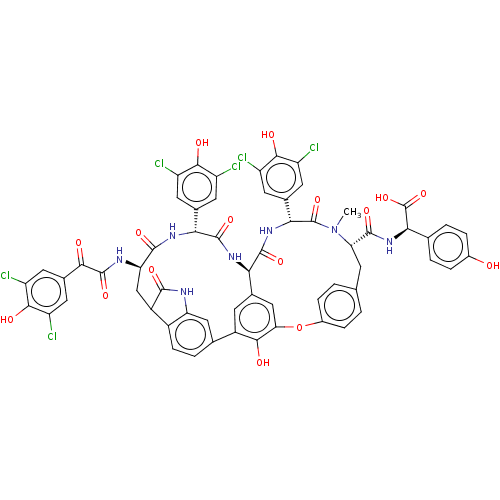 (CHEBI:65655 | COMPLESTATINS A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | J Nat Prod 64: 874-82 (2001) Article DOI: 10.1021/np000632z BindingDB Entry DOI: 10.7270/Q22N552N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50478735 (CHEBI:65655 | COMPLESTATINS A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3' processing/strand transfer coupled activity | J Nat Prod 64: 874-82 (2001) Article DOI: 10.1021/np000632z BindingDB Entry DOI: 10.7270/Q22N552N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||