

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50484341 CHEMBL1835497
SMILES: Cc1cccc(Cc2cc(C)ccc2OCCn2ccc(=O)[nH]c2=O)c1
InChI Key: InChIKey=FEGLBNQXLJPNTP-UHFFFAOYSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484341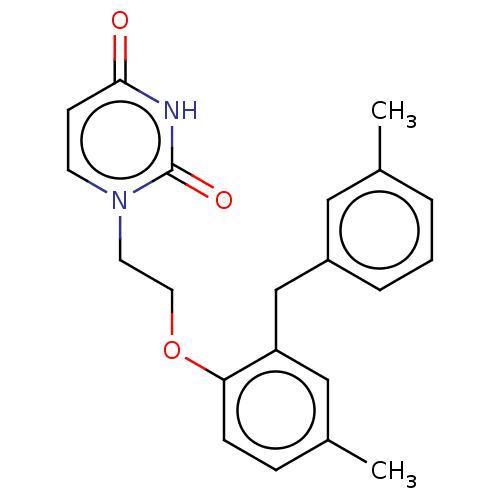 (CHEMBL1835497) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV1 reverse transcriptase p66/p51 heterodimer using [8-3H]dGTP as substrate after 30 mins by ... | Bioorg Med Chem 19: 5794-802 (2011) Article DOI: 10.1016/j.bmc.2011.08.025 BindingDB Entry DOI: 10.7270/Q2057JRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||