

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCc1c(CC)sc2nc([nH]c(=O)c12)-c1ccc(O)c(O)c1
InChI Key: InChIKey=HXAFMSQRYGOLLR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492106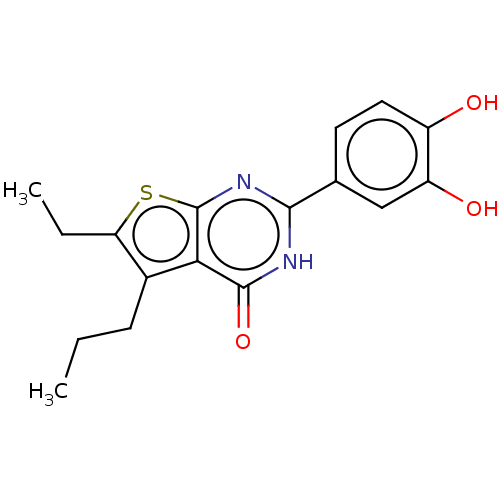 (CHEMBL2397401) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of RNase H activity of wild type HIV-1 reverse transcriptase using 18 nucleotide 3'-fluorescein-labeled RNA/18 nucleotide 5'-dabsyl-labele... | J Med Chem 56: 5436-45 (2013) Article DOI: 10.1021/jm400405z BindingDB Entry DOI: 10.7270/Q2B27Z7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||