

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50495258 CHEMBL3104556
SMILES: Cc1c(CC(=O)NCCO[N+]([O-])=O)cc(-c2ccc(cc2)S(C)(=O)=O)n1-c1ccc(F)cc1
InChI Key: InChIKey=IVQJGLCSKNJIAD-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50495258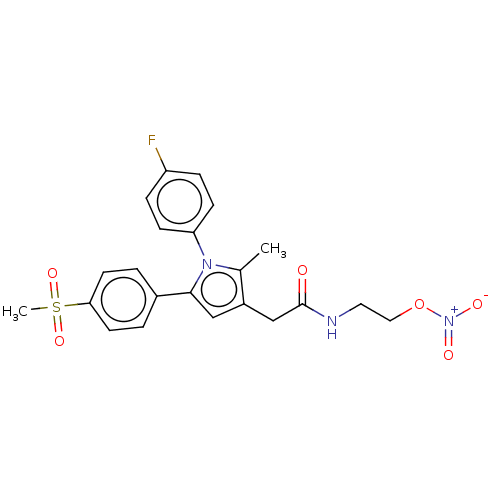 (CHEMBL3104556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of COX-1 in human whole blood assessed as thromboxane B2 production by RIA | Bioorg Med Chem 22: 772-86 (2014) Article DOI: 10.1016/j.bmc.2013.12.008 BindingDB Entry DOI: 10.7270/Q2K0777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50495258 (CHEMBL3104556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of COX-2 in human whole blood assessed as prostaglandin E2 production by RIA | Bioorg Med Chem 22: 772-86 (2014) Article DOI: 10.1016/j.bmc.2013.12.008 BindingDB Entry DOI: 10.7270/Q2K0777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Mus musculus) | BDBM50495258 (CHEMBL3104556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of COX-1 in mouse J774 cells using arachidonic acid as substrate assessed as inhibition of prostaglandin E2 production preincubated for 15... | Bioorg Med Chem 22: 772-86 (2014) Article DOI: 10.1016/j.bmc.2013.12.008 BindingDB Entry DOI: 10.7270/Q2K0777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Mus musculus (Mouse)) | BDBM50495258 (CHEMBL3104556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of LPS-induced COX-2 in mouse J774 cells using arachidonic acid as substrate assessed as inhibition of prostaglandin E2 production preincu... | Bioorg Med Chem 22: 772-86 (2014) Article DOI: 10.1016/j.bmc.2013.12.008 BindingDB Entry DOI: 10.7270/Q2K0777K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||