

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50500932 CHEMBL3798066
SMILES: CN(C)C(=O)c1cccc(c1)-c1cnc(N)c(n1)-c1nc2cccc(N3CCNCC3)c2[nH]1
InChI Key: InChIKey=CBAKEHNEEJTUGB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50500932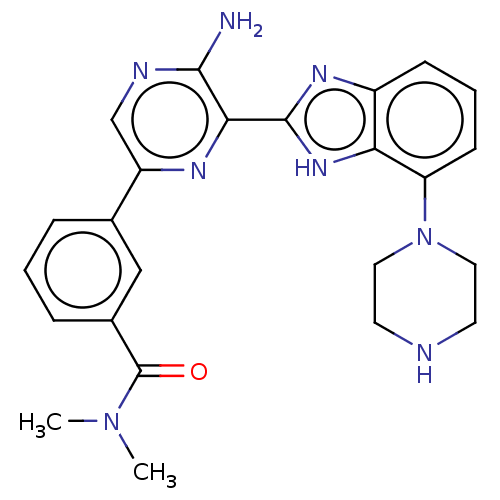 (CHEMBL3798066) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CDK1 (unknown origin) | J Med Chem 59: 3034-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01657 BindingDB Entry DOI: 10.7270/Q2RB77MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50500932 (CHEMBL3798066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ERK2-activated full length wild type MNK1a (unknown origin) using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 2 hrs by HTRF assay | J Med Chem 59: 3034-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01657 BindingDB Entry DOI: 10.7270/Q2RB77MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50500932 (CHEMBL3798066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CDK2 (unknown origin) | J Med Chem 59: 3034-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01657 BindingDB Entry DOI: 10.7270/Q2RB77MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM50500932 (CHEMBL3798066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human KMS11-luc cells assessed as inhibition of EIF4E phosphorylation at S209 after 3 hrs by quantitative electrochemilumines... | J Med Chem 59: 3034-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01657 BindingDB Entry DOI: 10.7270/Q2RB77MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50500932 (CHEMBL3798066) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged recombinant human full length wild type MNK2b using biotin-SGSGKRREILSRRPSYR-NH2 as substrate after 1 hr by HTRF... | J Med Chem 59: 3034-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01657 BindingDB Entry DOI: 10.7270/Q2RB77MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||