

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50505208 CHEMBL3311040
SMILES: COc1ccc(cc1OC)-c1coc2c3C=CC(C)(C)Oc3ccc2c1=O
InChI Key: InChIKey=BRFOUIGDZGRCOL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50505208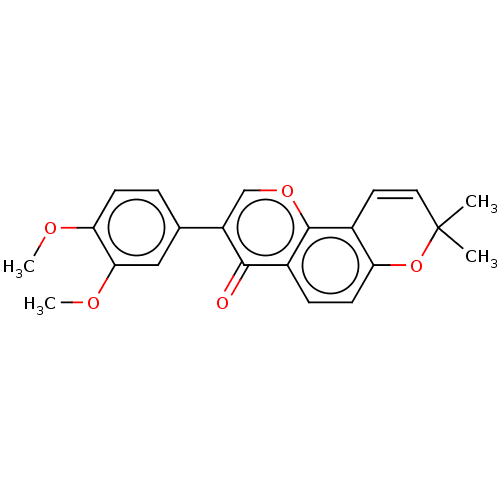 (CHEMBL3311040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Mixed type inhibition of BChE (unknown origin) using butyrylthiocholine iodide as substrate by Ellman's method based Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 29: 1194-1198 (2019) Article DOI: 10.1016/j.bmcl.2019.03.024 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50505208 (CHEMBL3311040) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 29: 1194-1198 (2019) Article DOI: 10.1016/j.bmcl.2019.03.024 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50505208 (CHEMBL3311040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Binding affinity to BChE (unknown origin) assessed as reduction in intrinsic protein fluorescence at 25 degC by fluorescence spectrophotometry | Bioorg Med Chem Lett 29: 1194-1198 (2019) Article DOI: 10.1016/j.bmcl.2019.03.024 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50505208 (CHEMBL3311040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 29: 1194-1198 (2019) Article DOI: 10.1016/j.bmcl.2019.03.024 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||