 Found 17 hits for monomerid = 50506158
Found 17 hits for monomerid = 50506158 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50506158
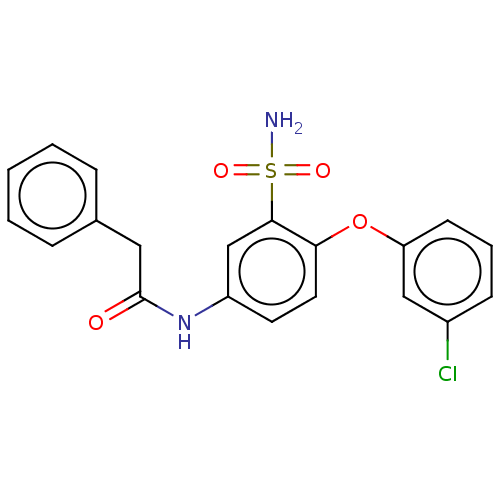
(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X4 receptor tranfected in human 1321N1 cells assessed as inhibition of Mg-ATP-induced calcium influx incubated for 30 ... |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate by LC-MS/MS analysis |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(RAT) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X4 receptor tranfected in human 1321N1 cells assessed as inhibition of Mg-ATP-induced calcium influx incubated for 30 mi... |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Mus musculus) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse P2X4 receptor tranfected in human 1321N1 cells assessed as inhibition of Mg-ATP-induced calcium influx incubated for 30 ... |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 1
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X1 receptor |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X3 receptor |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 2
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X2 receptor |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human DAT receptor by scintillation counting method |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate by LC-MS/MS analysis |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 4
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X4 receptor tranfected in human 1321N1 cells assessed as inhibition of inhibition of ATP-evoked current at -90 mV hold... |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50506158

(CHEMBL4521594)Show SMILES NS(=O)(=O)c1cc(NC(=O)Cc2ccccc2)ccc1Oc1cccc(Cl)c1 Show InChI InChI=1S/C20H17ClN2O4S/c21-15-7-4-8-17(12-15)27-18-10-9-16(13-19(18)28(22,25)26)23-20(24)11-14-5-2-1-3-6-14/h1-10,12-13H,11H2,(H,23,24)(H2,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 2 (unknown origin) |
J Med Chem 62: 11194-11217 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01304 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data