

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50509705 CHEMBL4444030
SMILES: Cl.NS(=O)(=O)c1ccc(cc1)-n1cc(COc2ccc(\C=N\Nc3c4CCCCc4nc4ccccc34)cc2)nn1
InChI Key: InChIKey=DVNIYUWNPGFHPL-KBVAKVRCSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50509705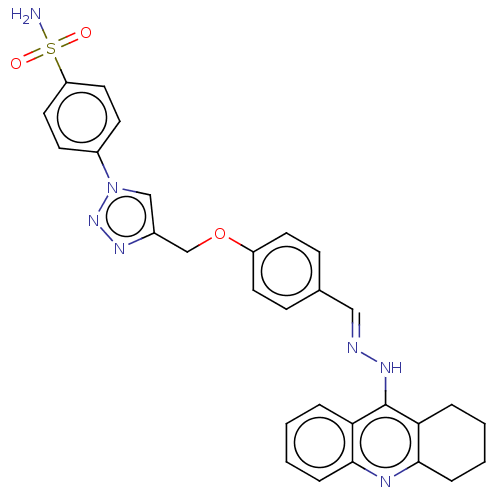 (CHEMBL4444030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for 180 ... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50509705 (CHEMBL4444030) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pre-incubated for 20 mins before substrate addition and measured for... | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50509705 (CHEMBL4444030) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 807 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 10 mins by spectrophotometry based Ellman's method | Eur J Med Chem 167: 161-186 (2019) Article DOI: 10.1016/j.ejmech.2019.02.012 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||