 Found 10 hits for monomerid = 50514245
Found 10 hits for monomerid = 50514245 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50514245
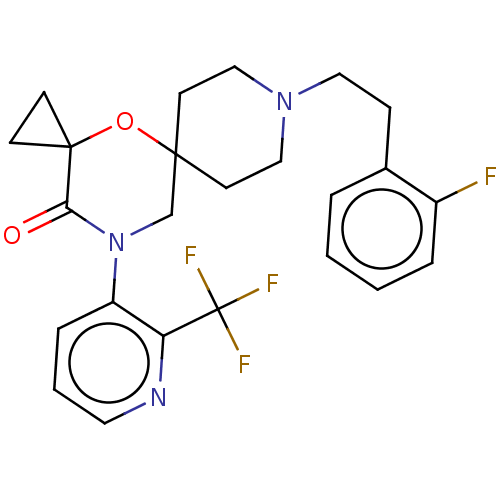
(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE Pharmaceuticals SA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from human sigma-1 receptor expressed in HEK293 membranes incubated for 120 mins by liquid scintillation countin... |
J Med Chem 63: 2434-2454 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01256 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE Pharmaceuticals SA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from human MOR expressed in CHOK1 cell membranes incubated for 60 mins by liquid scintillation counting method |
J Med Chem 63: 2434-2454 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01256 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE Pharmaceuticals SA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prazosin from human alpha1A adrenoreceptor expressed in enriched membranes incubated for 90 mins by liquid scintillation countin... |
J Med Chem 63: 2434-2454 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01256 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 754 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE Pharmaceuticals SA
Curated by ChEMBL
| Assay Description
Inhibition of 5HT2B receptor (unknown origin) |
J Med Chem 63: 2434-2454 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01256 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
ESTEVE Pharmaceuticals SA
Curated by ChEMBL
| Assay Description
Agonist activity at human MOR expressed in CHOK1 cells assessed as stimulation of cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 63: 2434-2454 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01256 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM50514245

(CHEMBL4461774)Show SMILES Fc1ccccc1CCN1CCC2(CC1)CN(C(=O)C1(CC1)O2)c1cccnc1C(F)(F)F Show InChI InChI=1S/C24H25F4N3O2/c25-18-5-2-1-4-17(18)7-13-30-14-10-22(11-15-30)16-31(21(32)23(33-22)8-9-23)19-6-3-12-29-20(19)24(26,27)28/h1-6,12H,7-11,13-16H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE Pharmaceuticals SA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHOK1 cells assessed as reduction in channel current at -80 mV holding potential by whole cell patch clamp assay |
J Med Chem 63: 2434-2454 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01256 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data