

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1cnnc(C)c1-c1ccc(Oc2nccc3occc23)cc1C
InChI Key: InChIKey=WTTAMIBXPRNVCC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450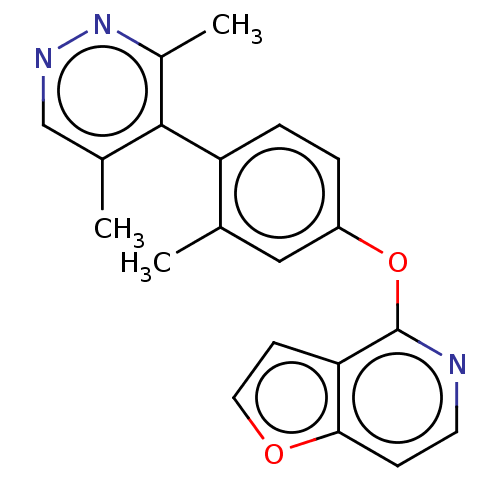 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Agonist activity at recombinant human D1 receptor expressed in HEK29T cells assessed as induction of stimulatory G-protein-mediated cAMP accumulation... | J Med Chem 62: 3753-3772 (2019) Article DOI: 10.1021/acs.jmedchem.9b00351 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Agonist activity at recombinant human D1 receptor expressed in HEK29T cells assessed as induction of stimulatory G-protein-mediated cAMP accumulation... | J Med Chem 62: 3753-3772 (2019) Article DOI: 10.1021/acs.jmedchem.9b00351 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||