

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50524988 CHEMBL4586773
SMILES: C[C@H](Nc1ccc2c(cn(-c3cc(C)n[nH]3)c2n1)C(=O)N1CCN(C)CC1)c1ccc(F)cn1
InChI Key: InChIKey=MFUMJAGTBAKMKI-INIZCTEOSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524988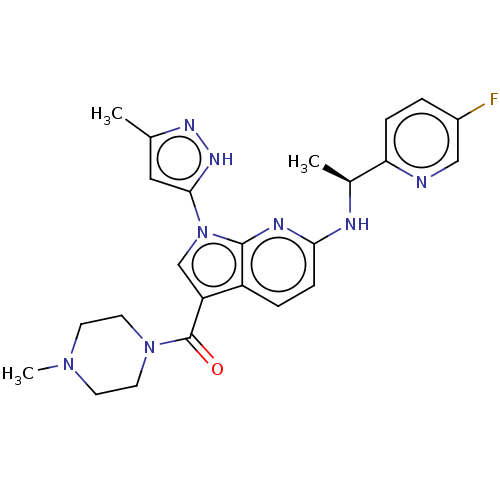 (CHEMBL4586773) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524988 (CHEMBL4586773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524988 (CHEMBL4586773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||