

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50526267 CHEMBL4444939
SMILES: N[C@H]1CC[C@@H](CC1)Nc1cc(Cl)nn2c(cnc12)-c1ccc2[nH]ncc2c1
InChI Key: InChIKey=ZNJUABQCCXFFKD-HDJSIYSDSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase/endoribonuclease IRE1 Alpha (Homo sapiens (Human)) | BDBM50526267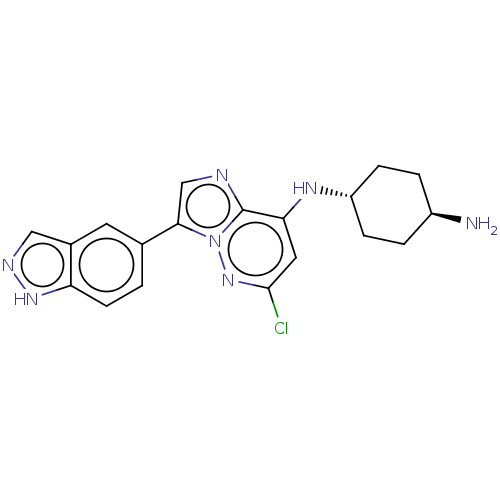 (CHEMBL4444939) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Inhibition of dephosphorylated IRE1alpha (G547 to L977 residues) (unknown origin) -dependent cleavage of FAM-labeled stem-loop RNA containing the XBP... | J Med Chem 62: 2447-2465 (2019) Article DOI: 10.1021/acs.jmedchem.8b01721 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 Alpha (Homo sapiens (Human)) | BDBM50526267 (CHEMBL4444939) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Inhibition of dephosphorylated IRE1alpha (G547 to L977 residues) (unknown origin) autophosphorylation at S724 after 25 mins in presence of ATP by dis... | J Med Chem 62: 2447-2465 (2019) Article DOI: 10.1021/acs.jmedchem.8b01721 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 Alpha (Homo sapiens (Human)) | BDBM50526267 (CHEMBL4444939) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Inhibition of tunicamycin-induced IRE1alpha (unknown origin) expressed in HEK293 cells assessed as reduction in IRE1alpha-dependent splicing of XBP1u... | J Med Chem 62: 2447-2465 (2019) Article DOI: 10.1021/acs.jmedchem.8b01721 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 Alpha (Homo sapiens (Human)) | BDBM50526267 (CHEMBL4444939) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester Curated by ChEMBL | Assay Description Displacement for LanthaScreen tracer from dephosphorylated IRE1alpha (G547 to L977 residues) (unknown origin) ATP binding site KEN domain after 60 mi... | J Med Chem 62: 2447-2465 (2019) Article DOI: 10.1021/acs.jmedchem.8b01721 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||