

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50527607 CHEMBL4443548
SMILES: CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(NCc3ccc(cn3)C(=O)NO)c2c(=O)n1-c1ccccc1
InChI Key: InChIKey=NNCNCNUUHFTRQN-FQEVSTJZSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50527607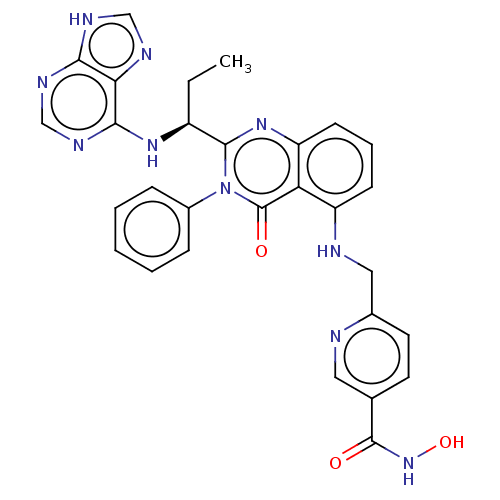 (CHEMBL4443548) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged HDAC3 (1 to 428 residues) expressed in baculovirus infected insect cells using fluo... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of full length recombinant human C-terminal FLAG/His-tagged HDAC1 (1 to 482 residues) expressed in baculovirus infected Sf21 insect cells ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-fusion tagged HDAC5 (657 to 1123 residues) expressed in baculovirus infected insect cells using fluoro... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using fluorogenic pep... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His-fusion tagged and N-terminal Streptavidin2-tagged human HDAC8 (1 to 377 residues) expressed in insect cells ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 10 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of HDAC10 (unknown origin) using fluorogenic peptide p53 residues 379-382 (RHKK(Ac)AMC) as substrate measured after 1 to 2 hrs by fluoresc... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Streptavidin2-tagged HDAC11 (1 to 347 residues) expressed in baculovirus infected insect cells using fluor... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kalpha assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma assessed as reduction in PIP3 product complex formation by measuring displacement of biotin-labelled PIP3 from complex ... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged and C-terminal His-tagged HDAC4 expressed in baculovirus infected insect cells using fluorogeni... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50527607 (CHEMBL4443548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC7 (518 to 991 residues) expressed in baculovirus infected insect cells using fluorogenic HD... | J Med Chem 63: 4256-4292 (2020) Article DOI: 10.1021/acs.jmedchem.0c00193 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||