

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50528418 CHEMBL4536780
SMILES: O[C@H]([C@@H](O)C(O)=O)C(O)=O.Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)NC1CNCCC1c1ccc(cc1)C(F)(F)F
InChI Key: InChIKey=LLSNFBIVFCQYNW-LREBCSMRSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50528418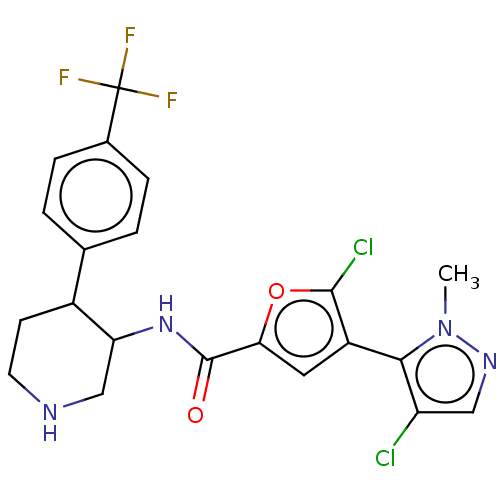 (CHEMBL4536780) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human Akt1 expressed in Escherichia coli using STK Substrate 2-biotin as substrate incubated for 1 hr by HTRF assay | J Med Chem 62: 7264-7288 (2019) Article DOI: 10.1021/acs.jmedchem.9b00891 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||