

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50529361 CHEMBL4515137::US10988487, Example 52
SMILES: OCCNC(=O)CNc1nonc1\C(N[C@H]1Cc2ccc(F)cc12)=N\O
InChI Key: InChIKey=OLPQNOYGDGMZSU-NSHDSACASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50529361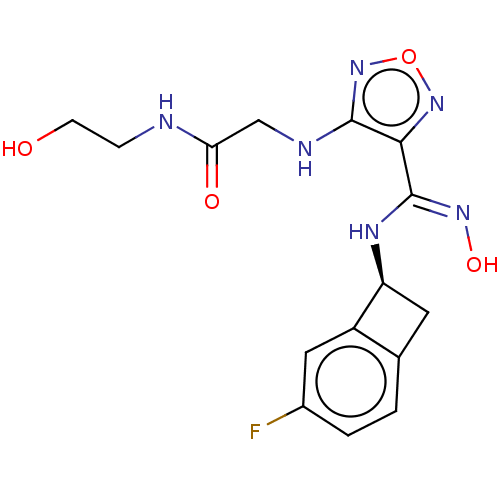 (CHEMBL4515137 | US10988487, Example 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of TDO in human SW480 cells incubated for 48 hrs using L-tryptophan as substrate by fluorescence based assay | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4B1 (Homo sapiens) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of CYP450 (unknown origin) | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 preincubated for 30 mins followed by substrate tryptophan addition and measured after 60 mins by fluorescence based assay | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged IDO1 (unknown origin) expressed in Escherichia coli preincubated for 30 mins followed by substrate tryptophan ad... | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 65.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description HIS-tagged IDO1 protein was recombinantly expressed in Escherichia coli using ZYP5052 autoinduction media supplemented with 500 μM delta aminole... | US Patent US10988487 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of IDO1 in human whole blood stimulated with IFNgamma/LPS assessed as unbound potency using L-tryptophan/kynurenine as substrate incubated... | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pregnane X receptor (Homo sapiens (Human)) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Activation of PXR (unknown origin) | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Rattus norvegicus) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | Reactome pathway UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of rat IDO1 preincubated for 30 mins followed by substrate tryptophan addition and measured after 60 mins by fluorescence based assay | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of IDO1 in human HeLa cells stimulated with IFNgamma using L-tryptophan as substrate incubated for 48 hrs by fluorescence based assay | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50529361 (CHEMBL4515137 | US10988487, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of IDO1 in human whole blood stimulated with IFNgamma/LPS using L-tryptophan/kynurenine as substrate incubated for 15 mins followed by IFN... | ACS Med Chem Lett 10: 1530-1536 (2019) Article DOI: 10.1021/acsmedchemlett.9b00344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||