

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50530252 CHEMBL4475081
SMILES: C[C@@H]1OCC2(CCN(CC2)c2cnc3c(c[nH]c3n2)-c2cccc(Cl)c2Cl)[C@@H]1N
InChI Key: InChIKey=SJLQMBIPYINRMF-HXPMCKFVSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens) | BDBM50530252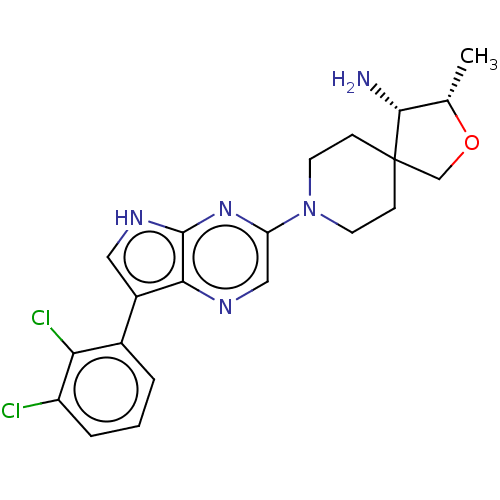 (CHEMBL4475081) | PDB KEGG GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 6His-tagged SHP2 (1 to 525 residues) expressed in Escherichia coli BL21 Star (DE3) using DiFMUP as substrate preincubated for 30 ... | J Med Chem 62: 1781-1792 (2019) Article DOI: 10.1021/acs.jmedchem.8b01725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens) | BDBM50530252 (CHEMBL4475081) | PDB KEGG GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of of SHP2 in human KYSE520 cells assessed as suppression of ERK phosphorylation after 2 hrs by AlphaScreen SureFire Phospho-ERK1/2 assay | J Med Chem 62: 1781-1792 (2019) Article DOI: 10.1021/acs.jmedchem.8b01725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens) | BDBM50530252 (CHEMBL4475081) | PDB KEGG GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of of SHP2 in human KYSE520 cells assessed as suppression of ERK phosphorylation after 2 hrs by AlphaScreen SureFire Phospho-ERK1/2 assay | J Med Chem 62: 1781-1792 (2019) Article DOI: 10.1021/acs.jmedchem.8b01725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens) | BDBM50530252 (CHEMBL4475081) | PDB KEGG GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human 6His-tagged SHP2 (1 to 525 residues) expressed in Escherichia coli BL21 Star (DE3) using DiFMUP as substrate preincubated for 30 ... | J Med Chem 62: 1781-1792 (2019) Article DOI: 10.1021/acs.jmedchem.8b01725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50530252 (CHEMBL4475081) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by Q-patch assay | J Med Chem 62: 1781-1792 (2019) Article DOI: 10.1021/acs.jmedchem.8b01725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50530252 (CHEMBL4475081) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by Q-patch assay | J Med Chem 62: 1781-1792 (2019) Article DOI: 10.1021/acs.jmedchem.8b01725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||