

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC1CCCN(CCCCCCOc2ccc(Cl)cc2)C1
InChI Key: InChIKey=LDQMGOJCUMEESA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50533196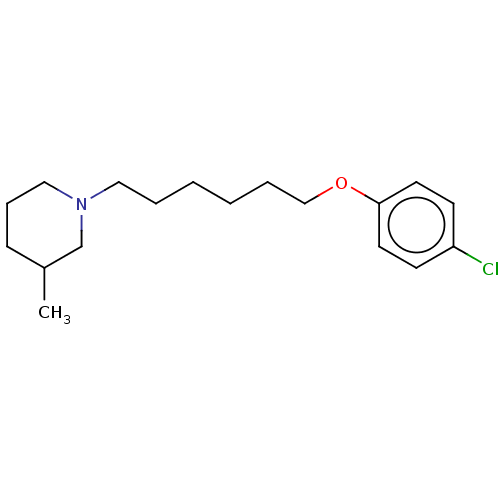 (CHEMBL3747900) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from human full length recombinant histamine H3 receptor expressed in HEK293 cell membranes after 90 mins ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50533196 (CHEMBL3747900) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine from human full length recombinant histamine H3 receptor expressed in HEK293 cell membranes after 90 mins ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50533196 (CHEMBL3747900) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 5 mins followed by substrate addition measured after ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50533196 (CHEMBL3747900) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50533196 (CHEMBL3747900) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50533196 (CHEMBL3747900) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 5 mins followed by substrate addition measured after ... | Bioorg Med Chem Lett 26: 4140-5 (2016) Article DOI: 10.1016/j.bmcl.2016.04.054 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||