

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50536168 CHEMBL4574111
SMILES: Cl.Nc1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(cc3)C3(N)CCC3)c2n1
InChI Key: InChIKey=UHXVRMFFZLLZNC-UHFFFAOYSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50536168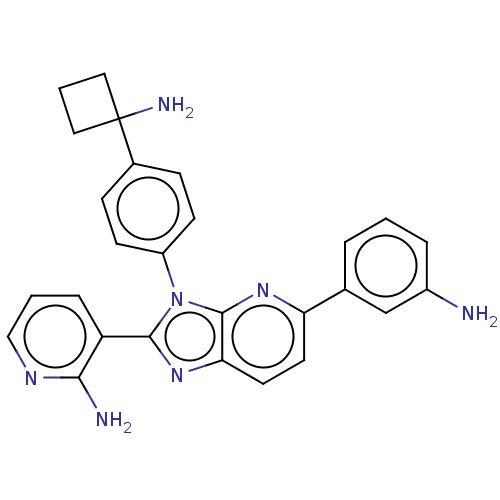 (CHEMBL4574111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 10 mins by LC/MS/MS analysis | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 10 mins by LC/MS/MS analysis | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate after 10 mins by LC/MS/MS analysis | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 10 mins by LC/MS/MS analysis | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins by LC/MS/MS analysis | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT3 (1 to 479 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT2 (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length active AKT2 (1 to 481 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-GRPRTSSFAE... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT2 (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT2 (1 to 481 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase AKT (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length active AKT3 (1 to 479 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-GRPRTSSFAE... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 10 mins by LC/MS/MS analysis | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50536168 (CHEMBL4574111) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule Inc. Curated by ChEMBL | Assay Description Inhibition of full length active AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-GRPRTSSFAE... | J Med Chem 59: 6455-69 (2016) Article DOI: 10.1021/acs.jmedchem.6b00619 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||