 Found 4 hits for monomerid = 50537741
Found 4 hits for monomerid = 50537741 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphoinositide 3-Kinase (PI3K), delta
(Homo sapiens (Human)) | BDBM50537741
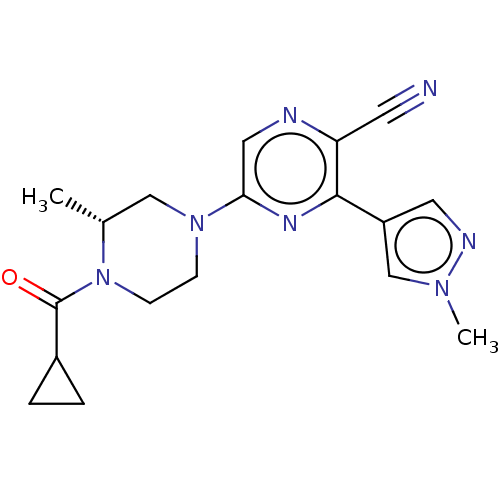
(CHEMBL4637209 | US11179389, Compound 1-18)Show SMILES C[C@@H]1CN(CCN1C(=O)C1CC1)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C18H21N7O/c1-12-10-24(5-6-25(12)18(26)13-3-4-13)16-9-20-15(7-19)17(22-16)14-8-21-23(2)11-14/h8-9,11-13H,3-6,10H2,1-2H3/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cytosolic c-Src |
Bioorg Med Chem Lett 30: (2020)
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (PIK3CD)(14-604)
(Homo sapiens (Human)) | BDBM50537741

(CHEMBL4637209 | US11179389, Compound 1-18)Show SMILES C[C@@H]1CN(CCN1C(=O)C1CC1)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C18H21N7O/c1-12-10-24(5-6-25(12)18(26)13-3-4-13)16-9-20-15(7-19)17(22-16)14-8-21-23(2)11-14/h8-9,11-13H,3-6,10H2,1-2H3/t12-/m1/s1 | GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (PIK3CD)(14-604)
(Homo sapiens (Human)) | BDBM50537741

(CHEMBL4637209 | US11179389, Compound 1-18)Show SMILES C[C@@H]1CN(CCN1C(=O)C1CC1)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C18H21N7O/c1-12-10-24(5-6-25(12)18(26)13-3-4-13)16-9-20-15(7-19)17(22-16)14-8-21-23(2)11-14/h8-9,11-13H,3-6,10H2,1-2H3/t12-/m1/s1 | GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50537741

(CHEMBL4637209 | US11179389, Compound 1-18)Show SMILES C[C@@H]1CN(CCN1C(=O)C1CC1)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C18H21N7O/c1-12-10-24(5-6-25(12)18(26)13-3-4-13)16-9-20-15(7-19)17(22-16)14-8-21-23(2)11-14/h8-9,11-13H,3-6,10H2,1-2H3/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of the fibroblast growth factor receptor (FGFR1) |
Bioorg Med Chem Lett 30: (2020)
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data