

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50538088 CHEMBL4640598
SMILES: C1CC(CCO1)n1cc(-c2ccncc2)c2ccncc12
InChI Key: InChIKey=NUTLDYDKKRBTHZ-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual-specificity tyrosine-phosphorylation regulated kinase 1A (Homo sapiens (Human)) | BDBM50538088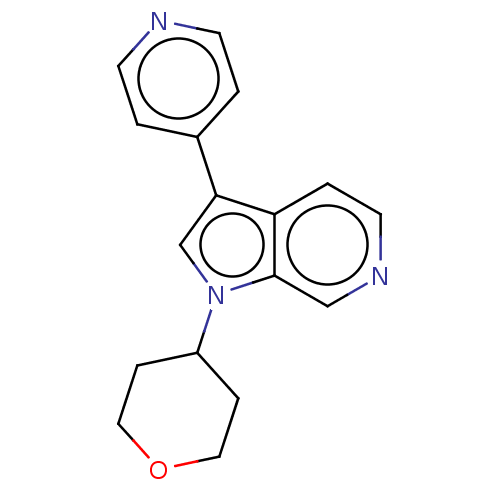 (CHEMBL4640598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50538088 (CHEMBL4640598) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human ERG by Qpatch S8 assay | J Med Chem 63: 2958-2973 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50538088 (CHEMBL4640598) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method | J Med Chem 63: 2958-2973 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50538088 (CHEMBL4640598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human His/GST-tagged GSK3beta (2 to 433 residues) expressed in baculovirus infected Sf9 cells using Ulight-glycogen synthas... | J Med Chem 63: 2958-2973 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||