

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50543396 CHEMBL4641012
SMILES: CCc1ccc(NC(=O)c2cc3cccc(O)c3oc2=N)cc1
InChI Key: InChIKey=HXXCNYPJYOGDGN-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto-reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543396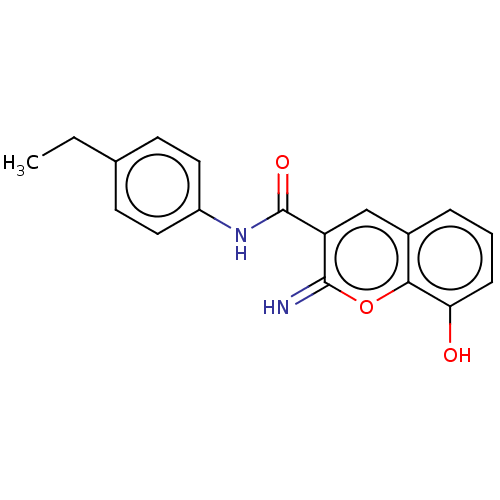 (CHEMBL4641012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor/Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50543396 (CHEMBL4641012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||