

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50545318 CHEMBL4647074
SMILES: Nc1nc(nc2sc(nc12)-c1ccco1)N1CCC(CNC(=O)c2ccc(cc2)S(N)(=O)=O)CC1
InChI Key: InChIKey=APLWPFYUEIDGFU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50545318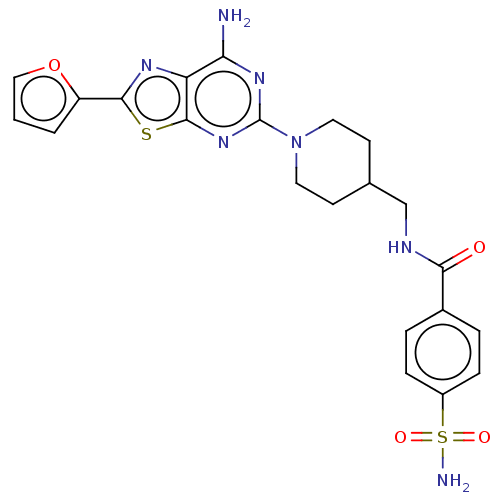 (CHEMBL4647074) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 488 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]ZM241383 from human A2AR expressed in CHO cells by competitive binding assay | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50545318 (CHEMBL4647074) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 receptor expressed in CHO cells by competitive binding assay | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50545318 (CHEMBL4647074) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]AB-MEGA from human A3R expressed in CHO cells by competitive binding assay | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptors; A2a & A2b (Homo sapiens (Human)) | BDBM50545318 (CHEMBL4647074) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human A2B receptor expressed in CHO cells assessed as reduction in cAMP production | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||