

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cccc(CNC(=O)C(\C)=C\CC\C(C)=C\CC\C(C)=C\CC[C@]2(C)CCc3cc(O)cc(C)c3O2)c1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555735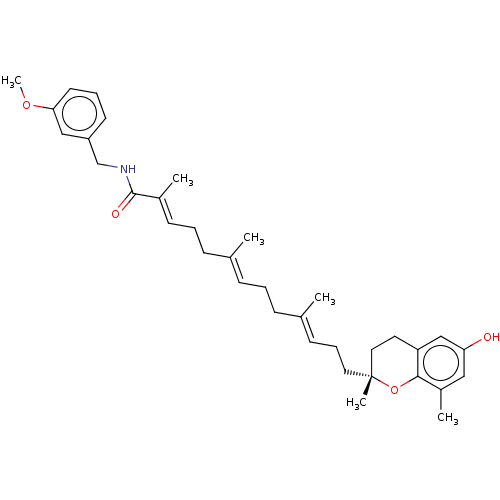 (CHEMBL4751589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555735 (CHEMBL4751589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||