

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Nc1cc(F)c(cc1C(=O)Nc1ccc(F)c(c1)C(F)F)S(=O)(=O)N1CCC(O)CC1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50559579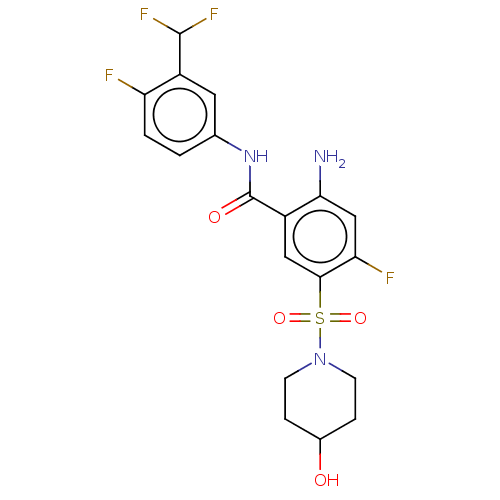 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP1A2 using phenacetin as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C9 using tolbutamide as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2D6 using dextromethorphan as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using sorafenib as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50559579 (CHEMBL4754334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C19 using S-mephenytoin as substrate incubated for 20 mins by LC-MS/MS with HPLC analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00606 BindingDB Entry DOI: 10.7270/Q2SJ1Q9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||