

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1cncc(c1)-c1ccnc3ccc-2cc13
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50570224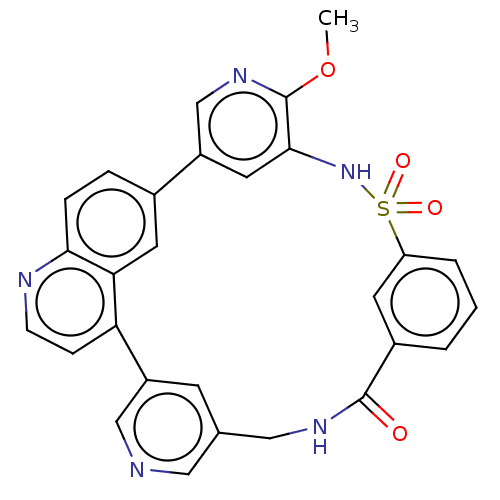 (CHEMBL4850101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length p110alpha (1 to 1068 residues) expressed in baculovirus-infected Sf21 cells by ADP-Hunter Plus assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50570224 (CHEMBL4850101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GST tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system measured by LanthaScreen assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50570224 (CHEMBL4850101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 (unknown origin) using Luciferin-ME as substrate at 10 uM preincubated with enzyme and substrate for 5 mins followed by addition... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50570224 (CHEMBL4850101) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by fluorescence polarization assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50570224 (CHEMBL4850101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 (unknown origin) using Luciferin-H as substrate at 10 uM preincubated with enzyme and substrate for 5 mins followed by addition ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens) | BDBM50570224 (CHEMBL4850101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 (unknown origin) using LuciferinH-EG as substrate at 10 uM preincubated with enzyme and substrate for 5 mins followed by additi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50570224 (CHEMBL4850101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 (unknown origin) using Luciferin-ME-EG as substrate at 10 uM preincubated with enzyme and substrate for 5 mins followed by addit... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50570224 (CHEMBL4850101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) using Luciferin-PPXE as substrate at 10 uM preincubated with enzyme and substrate for 5 mins followed by additi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113109 BindingDB Entry DOI: 10.7270/Q2JH3QZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||