

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(CCc2nc3cc(ccn3c2NC2CCCCC2)-c2c(C)noc2C)cc1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CREB-binding protein (Homo sapiens (Human)) | BDBM50571550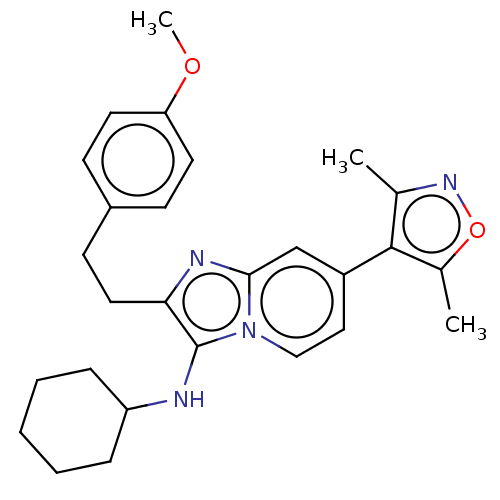 (CHEMBL4867046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged CBP (unknown origin) using biotinylated-H3K56ac peptide as substrate incubated for 1 hr by AlphaScreen assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02232 BindingDB Entry DOI: 10.7270/Q2NS0ZQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50571550 (CHEMBL4867046) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged BRD4 BD1 (unknown origin) using biotinylated-JQ1 peptide as substrate incubated for 1 hr by AlphaScreen assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02232 BindingDB Entry DOI: 10.7270/Q2NS0ZQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50571550 (CHEMBL4867046) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human partial length BRD4 bromodomain 1 long isoform (N44 to E168 residues) expressed in bacterial expression system by BromoELEC... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02232 BindingDB Entry DOI: 10.7270/Q2NS0ZQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50571550 (CHEMBL4867046) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human partial length EP300 (A1040 to G1161 residues) expressed in bacterial expression system by BromoELECT assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02232 BindingDB Entry DOI: 10.7270/Q2NS0ZQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50571550 (CHEMBL4867046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human partial length CREBBP (R1081 to G1197 residues) expressed in bacterial expression system by BromoELECT assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02232 BindingDB Entry DOI: 10.7270/Q2NS0ZQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||