 Found 5 hits for monomerid = 50598846
Found 5 hits for monomerid = 50598846 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598846
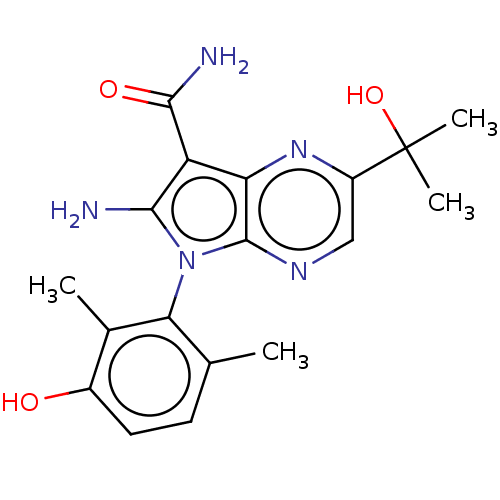
(CHEMBL5179743)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(cnc12)C(C)(C)O |(3.2,-2.6,;3.49,-1.4,;4.97,-.97,;5.34,.53,;4.22,1.59,;4.51,2.79,;2.74,1.16,;1.85,2.01,;2.38,-.34,;.88,-.66,;.25,-2.07,;.87,-3.13,;-1.28,-1.9,;-2.31,-3.05,;-3.51,-2.8,;-1.93,-4.22,;-1.6,-.4,;-2.93,.37,;-2.93,1.91,;-1.6,2.68,;-.27,1.91,;-.27,.37,;-4.27,2.68,;-5.34,2.07,;-4.27,4.22,;-5.34,3.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50598846

(CHEMBL5179743)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(cnc12)C(C)(C)O |(3.2,-2.6,;3.49,-1.4,;4.97,-.97,;5.34,.53,;4.22,1.59,;4.51,2.79,;2.74,1.16,;1.85,2.01,;2.38,-.34,;.88,-.66,;.25,-2.07,;.87,-3.13,;-1.28,-1.9,;-2.31,-3.05,;-3.51,-2.8,;-1.93,-4.22,;-1.6,-.4,;-2.93,.37,;-2.93,1.91,;-1.6,2.68,;-.27,1.91,;-.27,.37,;-4.27,2.68,;-5.34,2.07,;-4.27,4.22,;-5.34,3.3,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598846

(CHEMBL5179743)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(cnc12)C(C)(C)O |(3.2,-2.6,;3.49,-1.4,;4.97,-.97,;5.34,.53,;4.22,1.59,;4.51,2.79,;2.74,1.16,;1.85,2.01,;2.38,-.34,;.88,-.66,;.25,-2.07,;.87,-3.13,;-1.28,-1.9,;-2.31,-3.05,;-3.51,-2.8,;-1.93,-4.22,;-1.6,-.4,;-2.93,.37,;-2.93,1.91,;-1.6,2.68,;-.27,1.91,;-.27,.37,;-4.27,2.68,;-5.34,2.07,;-4.27,4.22,;-5.34,3.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50598846

(CHEMBL5179743)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(cnc12)C(C)(C)O |(3.2,-2.6,;3.49,-1.4,;4.97,-.97,;5.34,.53,;4.22,1.59,;4.51,2.79,;2.74,1.16,;1.85,2.01,;2.38,-.34,;.88,-.66,;.25,-2.07,;.87,-3.13,;-1.28,-1.9,;-2.31,-3.05,;-3.51,-2.8,;-1.93,-4.22,;-1.6,-.4,;-2.93,.37,;-2.93,1.91,;-1.6,2.68,;-.27,1.91,;-.27,.37,;-4.27,2.68,;-5.34,2.07,;-4.27,4.22,;-5.34,3.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50598846

(CHEMBL5179743)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(cnc12)C(C)(C)O |(3.2,-2.6,;3.49,-1.4,;4.97,-.97,;5.34,.53,;4.22,1.59,;4.51,2.79,;2.74,1.16,;1.85,2.01,;2.38,-.34,;.88,-.66,;.25,-2.07,;.87,-3.13,;-1.28,-1.9,;-2.31,-3.05,;-3.51,-2.8,;-1.93,-4.22,;-1.6,-.4,;-2.93,.37,;-2.93,1.91,;-1.6,2.68,;-.27,1.91,;-.27,.37,;-4.27,2.68,;-5.34,2.07,;-4.27,4.22,;-5.34,3.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data