Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50606763
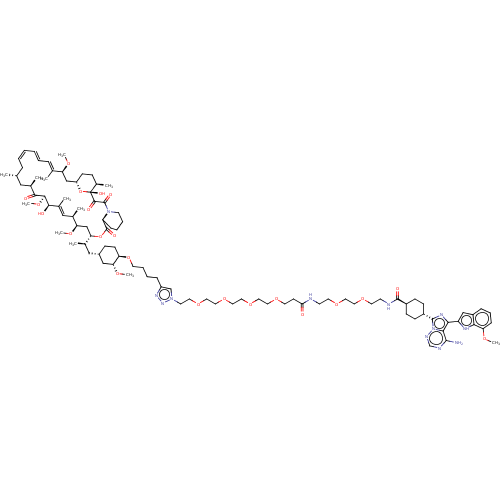
(CHEMBL5219551)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@@]([H])(C[C@@H](OC)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)C\C=C/C=C/C=C(C)/[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCCCCc2cn(CCOCCOCCOCCOCCC(=O)NCCOCCOCCNC(=O)[C@H]3CC[C@@H](CC3)c3nc(-c4cc5cccc(OC)c5[nH]4)c4c(N)ncnn34)nn2)[C@@H](C1)OC |r,wU:18.20,57.60,60.62,129.140,22.24,35.37,40.42,1.0,4.4,6.6,25.27,103.106,wD:63.66,28.30,43.45,53.57,33.35,100.102,c:32,48,52,t:50,(-26.38,-7.52,;-27.6,-6.59,;-28.89,-5.74,;-28.77,-4.28,;-27.41,-3.59,;-27.33,-2.05,;-26.16,-4.36,;-25.96,-2.83,;-26.24,-5.9,;-24.88,-3.71,;-24.76,-2.25,;-23.59,-4.55,;-23.71,-6.01,;-22.31,-3.91,;-22.24,-2.37,;-20.87,-1.67,;-19.58,-2.52,;-19.7,-3.97,;-21.07,-4.67,;-19.85,-5.61,;-18.43,-5.02,;-20.05,-7.13,;-18.83,-8.07,;-17.61,-9.01,;-19.03,-9.6,;-17.81,-10.54,;-16.39,-9.95,;-15.17,-10.89,;-18.1,-12.05,;-16.93,-13.05,;-19.55,-12.56,;-19.83,-14.08,;-18.66,-15.08,;-21.29,-14.59,;-22.45,-13.58,;-21.57,-16.1,;-20.4,-17.1,;-20.69,-18.62,;-22.7,-17.7,;-22.12,-19.12,;-24.42,-17.77,;-25.01,-16.35,;-25.36,-18.99,;-26.89,-18.79,;-27.82,-20.01,;-27.47,-17.37,;-29,-17.16,;-29.59,-15.74,;-28.65,-14.52,;-29.24,-13.1,;-28.3,-11.87,;-28.89,-10.45,;-30.42,-10.25,;-27.95,-9.23,;-28.54,-7.81,;-26.42,-9.43,;-25.49,-8.21,;-17.41,-7.48,;-16.19,-8.42,;-17.2,-5.96,;-15.78,-5.37,;-14.56,-6.31,;-13.14,-5.72,;-12.93,-4.19,;-11.51,-3.6,;-10.29,-4.54,;-8.87,-3.95,;-7.64,-4.89,;-7.85,-6.42,;-6.63,-7.36,;-5.11,-6.86,;-4.24,-8.13,;-2.71,-8.32,;-1.78,-7.1,;-.25,-7.29,;.68,-6.07,;2.21,-6.26,;3.14,-5.04,;4.67,-5.23,;5.6,-4.01,;7.13,-4.2,;8.06,-2.98,;9.59,-3.17,;10.52,-1.95,;12.05,-2.14,;12.98,-.92,;14.51,-1.11,;15.11,-2.53,;15.44,.11,;16.97,-.08,;17.9,1.14,;19.43,.95,;20.36,2.18,;21.89,1.98,;22.82,3.21,;24.35,3.01,;25.28,4.24,;26.81,4.04,;27.74,5.27,;29.27,5.07,;27.15,6.69,;28.08,7.91,;27.49,9.33,;25.96,9.53,;25.03,8.3,;25.62,6.88,;25.36,10.95,;26.16,12.26,;25.16,13.43,;25.51,14.93,;24.51,16.1,;25.31,17.41,;24.86,18.89,;25.92,20.01,;27.42,19.66,;27.86,18.18,;29.36,17.83,;30.42,18.95,;26.81,17.06,;26.93,15.53,;23.74,12.84,;22.35,13.49,;22.08,15.01,;21.08,12.62,;21.21,11.08,;22.6,10.42,;23.86,11.3,;-5.17,-9.35,;-6.62,-8.84,;-14.16,-3.25,;-15.58,-3.84,;-13.95,-1.73,;-15.17,-.79,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <32 | n/a | n/a | n/a | n/a | n/a | n/a |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
