

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [#7]-[#6](=O)-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-c2ccccc2-[#6]-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@H]-1-[#6][Se;v2][Se;v2][#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](-[#7])=O
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50613532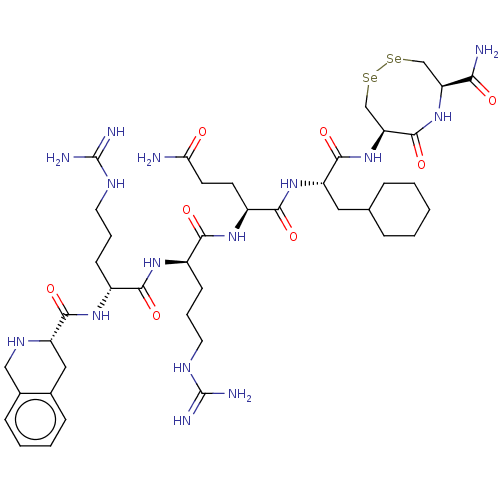 (CHEMBL5288155) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||