

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)n1cnc2cc(nc(Nc3ccncc3F)c12)-c1ccc2c(c1)N(C1CC(C1)N1CC3CCC(C3)C1)C(=O)C21CCN(CC1)C(=O)C1CCO1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM509018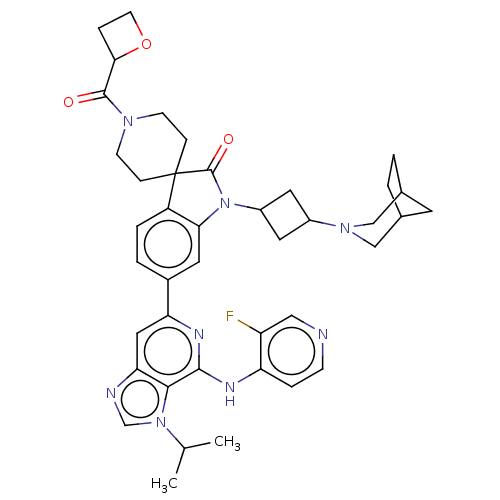 (US11071730, Example 125) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Recombinant HPK1 kinase domain produced via baculovirus infection of insect cells was obtained from Proteros (Proteros Biostructures #PR-0322) and wa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RR22CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||