

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM7991 (3S,4S)-5-(4-Bromobenzyloxy)-3-hydroxy-4-(2-thiophen-2-ylacetylamino)pentanoic Acid [(S)-1-((S)-1-Carbamoyl-3-methylbutylcarbamoyl)ethyl]amide::(3S,4S)-5-[(4-bromophenyl)methoxy]-N-[(1S)-1-{[(1S)-1-carbamoyl-3-methylbutyl]carbamoyl}ethyl]-3-hydroxy-4-[1-(thiophen-2-yl)acetamido]pentanamide::Statine-like inhibitor 30
SMILES: CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](COCc1ccc(Br)cc1)NC(=O)Cc1cccs1)C(N)=O
InChI Key: InChIKey=FIVRWDQFTCJMDY-ZMVGRULKSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin I (Plasmodium falciparum) | BDBM7991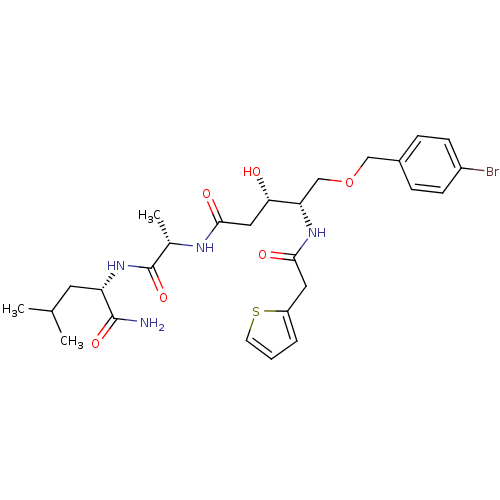 ((3S,4S)-5-(4-Bromobenzyloxy)-3-hydroxy-4-(2-thioph...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM7991 ((3S,4S)-5-(4-Bromobenzyloxy)-3-hydroxy-4-(2-thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin 2 (Plasmodium falciparum) | BDBM7991 ((3S,4S)-5-(4-Bromobenzyloxy)-3-hydroxy-4-(2-thioph...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linkoping University | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 47: 3353-66 (2004) Article DOI: 10.1021/jm031106i BindingDB Entry DOI: 10.7270/Q2WM1BMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||