

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9488 US8541427, 1
SMILES: COc1nc(nc(C)c1F)N1C[C@H]2C(=O)N(C)C(=N)N[C@]2(C1)c1ccc(F)cc1F
InChI Key: InChIKey=FNMJFZYRSQTKIH-HXPMCKFVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM9488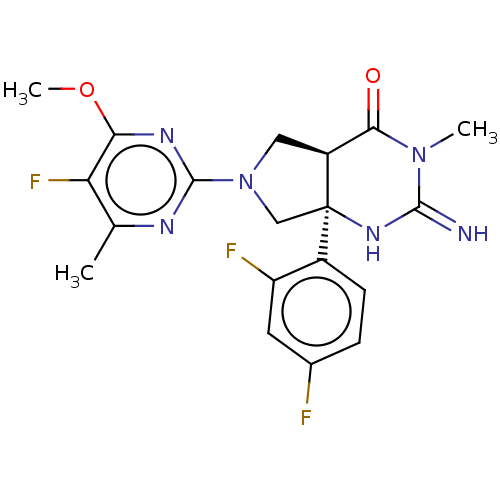 (US8541427, 1) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 4.70 | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme, Corp. US Patent | Assay Description A homogeneous time-resolved FRET assay was used to determine IC50 values for inhibitors of the soluble human BACE1 catalytic domain. The assay monit... | US Patent US8541427 (2013) BindingDB Entry DOI: 10.7270/Q26D5RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... | J Med Chem 59: 3231-48 (2016) BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human histamine H2 receptor | J Med Chem 59: 3231-48 (2016) BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human motilin receptor | J Med Chem 59: 3231-48 (2016) BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK293 cells transfected with human APP Swe/Lon mutations assessed as amyloid beta 40 level after 4 hrs by electrochemil... | J Med Chem 59: 3231-48 (2016) BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM9488 (US8541427, 1) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 59: 3231-48 (2016) BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM9488 (US8541427, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone and midazolam as substrate preincubated for 30 mins followed substrate addition by ... | J Med Chem 59: 3231-48 (2016) BindingDB Entry DOI: 10.7270/Q2CR5W8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||