

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50361396 (S)-(+)-Decursin::DECURSIN
SMILES: [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#8]-[#6@H]1-[#6]-c2cc3ccc(=O)oc3cc2-[#8]C1([#6])[#6]
InChI Key: InChIKey=CUKSFECWKQBVED-INIZCTEOSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50361396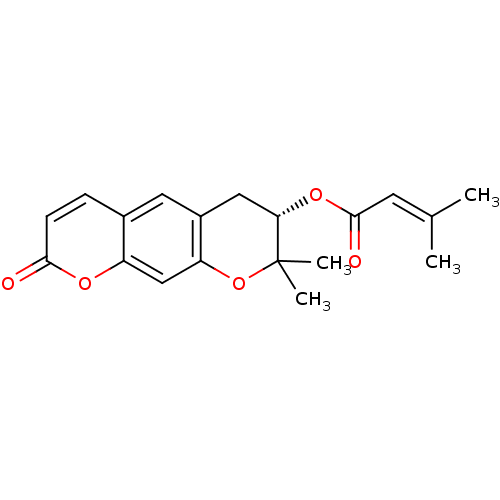 ((S)-(+)-Decursin | DECURSIN) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assay | Bioorg Med Chem 20: 784-8 (2012) Article DOI: 10.1016/j.bmc.2011.12.002 BindingDB Entry DOI: 10.7270/Q2F47PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50361396 ((S)-(+)-Decursin | DECURSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of AChE by spectrophotometry | J Nat Prod 64: 683-5 (2001) BindingDB Entry DOI: 10.7270/Q29G5NQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50361396 ((S)-(+)-Decursin | DECURSIN) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine as substrate after 10 mins | Bioorg Med Chem Lett 29: 839-843 (2019) Article DOI: 10.1016/j.bmcl.2019.01.016 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50361396 ((S)-(+)-Decursin | DECURSIN) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using kynuramine as substrate preincubated for 30 mins followed by substrate addition | Bioorg Med Chem Lett 28: 584-588 (2018) Article DOI: 10.1016/j.bmcl.2018.01.049 BindingDB Entry DOI: 10.7270/Q25T3P2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50361396 ((S)-(+)-Decursin | DECURSIN) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using kynuramine as substrate incubated for 10 mins by spectrophotometric method | Bioorg Med Chem Lett 28: 2403-2407 (2018) Article DOI: 10.1016/j.bmcl.2018.06.023 BindingDB Entry DOI: 10.7270/Q23R0WCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||