 Found 12 hits for monomerid = 50209683
Found 12 hits for monomerid = 50209683 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683
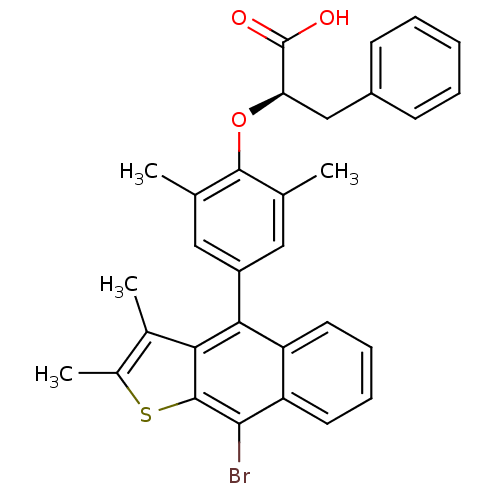
((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B after 10 mins |
Bioorg Med Chem Lett 17: 2760-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.069
BindingDB Entry DOI: 10.7270/Q2445N91 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 17: 2728-30 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.001
BindingDB Entry DOI: 10.7270/Q2W958WN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of IKKbeta after 30 mins |
Bioorg Med Chem Lett 17: 2728-30 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.001
BindingDB Entry DOI: 10.7270/Q2W958WN |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 19: 6161-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.020
BindingDB Entry DOI: 10.7270/Q20P102S |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B (1 to 299 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 30 mins followed by ... |
J Nat Prod 83: 1598-1610 (2020)
|
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B after 10 mins |
Bioorg Med Chem Lett 17: 2760-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.069
BindingDB Entry DOI: 10.7270/Q2445N91 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B after 10 mins |
Bioorg Med Chem Lett 17: 5357-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.019
BindingDB Entry DOI: 10.7270/Q2BK1D55 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP hydrolase assay |
Eur J Med Chem 44: 3280-4 (2009)
Article DOI: 10.1016/j.ejmech.2009.02.011
BindingDB Entry DOI: 10.7270/Q2JH3N4J |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PTP1B (1 to 299) expressed in Escherichia coli using p-nitrophenyl phosphate as substrate |
Bioorg Med Chem 23: 2786-97 (2015)
Article DOI: 10.1016/j.bmc.2015.03.075
BindingDB Entry DOI: 10.7270/Q2BG2QQD |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50209683

((R)-2-(4-(9-bromo-2,3-dimethylnaphtho[2,3-b]thioph...)Show SMILES Cc1sc2c(Br)c3ccccc3c(-c3cc(C)c(O[C@H](Cc4ccccc4)C(O)=O)c(C)c3)c2c1C Show InChI InChI=1S/C31H27BrO3S/c1-17-14-22(15-18(2)29(17)35-25(31(33)34)16-21-10-6-5-7-11-21)27-23-12-8-9-13-24(23)28(32)30-26(27)19(3)20(4)36-30/h5-15,25H,16H2,1-4H3,(H,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical Industries
Curated by ChEMBL
| Assay Description
Inhibition of PTB1B using pNPP as substrate after 30 mins |
Bioorg Med Chem 20: 1060-75 (2012)
Article DOI: 10.1016/j.bmc.2011.11.035
BindingDB Entry DOI: 10.7270/Q2BC3ZZS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data