

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50191348 (3alpha,5beta)-3-hydroxyandrostan-17-one::3alpha-etiocholanolone::3alpha-hydroxy-5beta-androstan-17-one::5-isoandrosterone::5beta-androstan-3alpha-ol-17-one::5beta-androsterone::CHEMBL85799::Etiocholanolone
SMILES: C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CCC2=O
InChI Key: InChIKey=QGXBDMJGAMFCBF-BNSUEQOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticosteroid-binding globulin (Homo sapiens) | BDBM50191348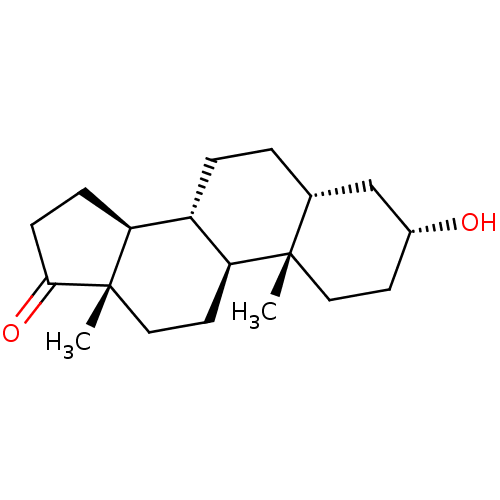 ((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Zoki Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Binding affinity to human CBG receptor (corticosteroid-binding globulins) | J Med Chem 47: 2732-42 (2004) Article DOI: 10.1021/jm030364c BindingDB Entry DOI: 10.7270/Q2WM1H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50191348 ((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description The ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity in transfected human embryonic kidney (HEK)-293 cells experiment 1 | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50191348 ((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval Curated by ChEMBL | Assay Description The ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity in transfected human embryonic kidney (HEK)-293 cells experiment 3 | J Med Chem 45: 640-53 (2002) BindingDB Entry DOI: 10.7270/Q2K93881 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sex hormone-binding globulin (Homo sapiens (Human)) | BDBM50191348 ((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Displacement of [3H]5alpha dihydrotestosterone from human sex hormone binding globulin | J Med Chem 51: 2047-56 (2008) Article DOI: 10.1021/jm7011485 BindingDB Entry DOI: 10.7270/Q2RX9DC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled bile acid receptor 1 (Homo sapiens (Human)) | BDBM50191348 ((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.76E+3 | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Agonist activity at human TGR5 expressed in CHO cells by luciferase assay | J Med Chem 51: 1831-41 (2008) Article DOI: 10.1021/jm7015864 BindingDB Entry DOI: 10.7270/Q2222VNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50191348 ((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) and Laval University Curated by ChEMBL | Assay Description Inhibitory concentration against type-3 17-beta-HSD expressed in HEK293 cells | Bioorg Med Chem Lett 10: 2533-6 (2001) BindingDB Entry DOI: 10.7270/Q2GH9JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50191348 ((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Universit£ Laval Curated by ChEMBL | Assay Description Inhibition of type-3 17 beta-hydroxysteroid dehydrogenase expressed in HEK293 cells at 37 degree C pH7.4 | J Med Chem 48: 5257-68 (2005) Article DOI: 10.1021/jm058179h BindingDB Entry DOI: 10.7270/Q21C1XNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||