

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50259708 BAY-2502::BAY-A2502::BAYER-2502::CHEBI:7566::DNDI1613515::Lampit::NIFURTIMOX::Nifurtimox
SMILES: CC1CS(=O)(=O)CCN1\N=C\c1ccc(o1)[N+]([O-])=O
InChI Key: InChIKey=ARFHIAQFJWUCFH-IZZDOVSWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259708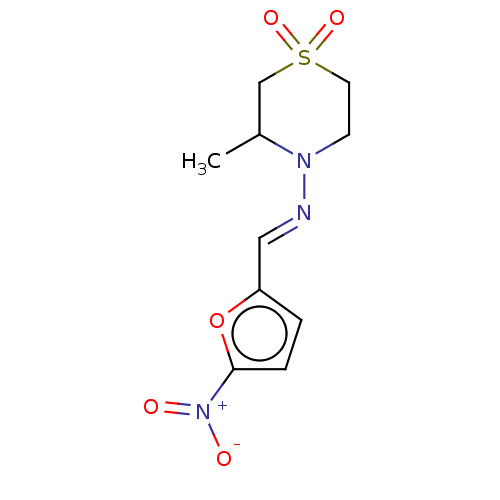 (BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 10 kDa chaperonin (Escherichia coli) | BDBM50259708 (BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL/GroES-ATPase activity expressed in Escherichia coli DH5alpha/BL21 (DE3) assessed as inhibition of denatured MDH ... | Bioorg Med Chem Lett 26: 5247-5253 (2016) Article DOI: 10.1016/j.bmcl.2016.09.051 BindingDB Entry DOI: 10.7270/Q2WW7MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HSP60/HSP10 (Homo sapiens) | BDBM50259708 (BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of human mitochondrial HSP60/HSP10-ATPase activity expressed in Escherichia coli DH5alpha/BL21 (DE3) assessed as inhibition of denatured M... | Bioorg Med Chem Lett 26: 5247-5253 (2016) Article DOI: 10.1016/j.bmcl.2016.09.051 BindingDB Entry DOI: 10.7270/Q2WW7MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HSP60/HSP10 (Homo sapiens) | BDBM50259708 (BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of human mitochondrial HSP60/HSP10 expressed in Escherichia coli DH5alpha/BL21 (DE3) assessed as inhibition of denatured MDH refolding pre... | Bioorg Med Chem Lett 26: 5247-5253 (2016) Article DOI: 10.1016/j.bmcl.2016.09.051 BindingDB Entry DOI: 10.7270/Q2WW7MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 10 kDa chaperonin (Escherichia coli) | BDBM50259708 (BAY-2502 | BAY-A2502 | BAYER-2502 | CHEBI:7566 | D...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL/GroES expressed in Escherichia coli DH5alpha/BL21 (DE3) assessed as inhibition of denatured MDH refolding preinc... | Bioorg Med Chem Lett 26: 5247-5253 (2016) Article DOI: 10.1016/j.bmcl.2016.09.051 BindingDB Entry DOI: 10.7270/Q2WW7MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||