

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50418089 LIMONIN::Limonin
SMILES: [H][C@]12O[C@@]11[C@@](C)(CC[C@]3([H])[C@@]45COC(=O)C[C@]4([H])OC(C)(C)[C@]5([H])CC(=O)[C@@]13C)[C@@H](OC2=O)c1ccoc1
InChI Key: InChIKey=BEAROWPOUPFNRD-HEZIXOHUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50418089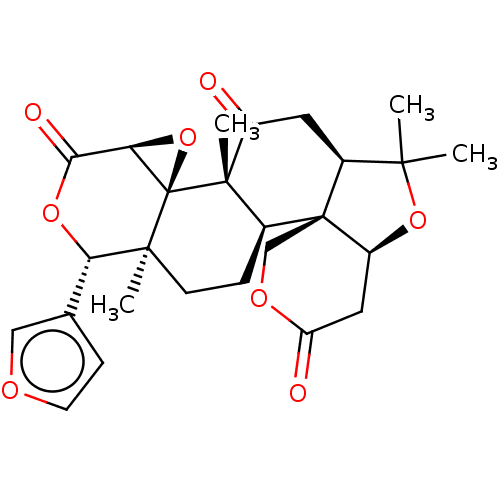 (LIMONIN | Limonin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycin | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein 1/Multidrug resistance associated protein 1 (Homo sapiens (Human)) | BDBM50418089 (LIMONIN | Limonin) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Punjab Curated by ChEMBL | Assay Description Inhibition of P-gp in human Caco2 cells assessed as potentiation of doxorubicin-induced cytotoxicity by measuring reduction in doxorubicin IC50 at 2.... | Eur J Med Chem 176: 268-291 (2019) Article DOI: 10.1016/j.ejmech.2019.05.027 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50418089 (LIMONIN | Limonin) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Inhibition of HIV1 RT | J Nat Prod 54: 143-54 Article DOI: 10.1021/np50073a012 BindingDB Entry DOI: 10.7270/Q2NK3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50418089 (LIMONIN | Limonin) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata Curated by ChEMBL | Assay Description Inhibition of HIV1 3B reverse transcriptase activity | Bioorg Med Chem 19: 2084-9 (2011) Article DOI: 10.1016/j.bmc.2011.01.024 BindingDB Entry DOI: 10.7270/Q2CN76Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50418089 (LIMONIN | Limonin) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome Tor Vergata Curated by ChEMBL | Assay Description Inhibition of HIV1 3B reverse transcriptase activity infected in human H9 cells assessed as level of p24 antigen | Bioorg Med Chem 19: 2084-9 (2011) Article DOI: 10.1016/j.bmc.2011.01.024 BindingDB Entry DOI: 10.7270/Q2CN76Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||