 Found 5 hits for monomerid = 50317209
Found 5 hits for monomerid = 50317209 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209
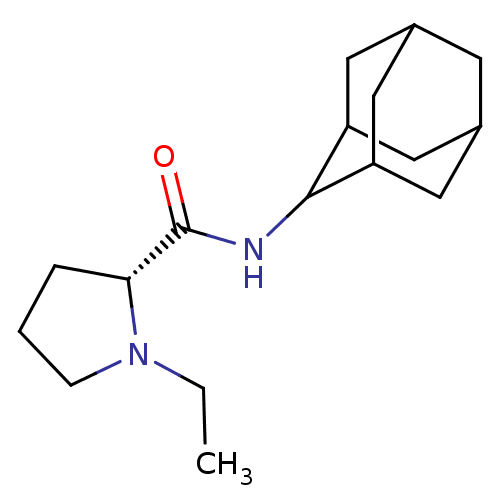
((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase
(Homo sapiens (Human)) | BDBM50317209

((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes assessed as conversion of [3H]-cortisone to [3H]-cortisol after 30 mins by scintillation proximit... |
Bioorg Med Chem 23: 7607-17 (2015)
Article DOI: 10.1016/j.bmc.2015.11.004
BindingDB Entry DOI: 10.7270/Q2VH5RVR |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209

((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using [3H]cortisone as substrate preincubated for 15 mins followed by substrate addition and meas... |
Eur J Med Chem 139: 412-428 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.003
BindingDB Entry DOI: 10.7270/Q2JM2D5R |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209

((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 expressed in HEK293 cells co-transfected with GRE-luciferase after 6 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317209

((2R)-N-(adamantan-2-yl)-1-ethylpyrrolidine-2-carbo...)Show SMILES CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:6.7,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:9:10:14.15.17:19,10:11:14:17.18.19,10:18:14:12.16.11,(-6.05,6.18,;-5.42,4.76,;-6.33,3.51,;-7.87,3.49,;-8.34,2.03,;-7.09,1.12,;-5.88,2.1,;-4.36,1.59,;-3,2.34,;-4.38,.05,;-5.73,-.7,;-5.74,-2.24,;-7.15,-2.6,;-8.49,-2.1,;-9.69,-3.39,;-8.18,-2.96,;-6.76,-3.53,;-8.19,-1.37,;-7.14,-.12,;-8.5,-.6,)| Show InChI InChI=1S/C17H28N2O/c1-2-19-5-3-4-15(19)17(20)18-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,2-10H2,1H3,(H,18,20)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 expressed in HEK cells |
ACS Med Chem Lett 3: 793-798 (2012)
Article DOI: 10.1021/ml300144n
BindingDB Entry DOI: 10.7270/Q2K938NZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data