

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50093003 (4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pentanoylsulfamoyl)-acetylamino]-4-carbamoyl-6-hydroxy-2-methyl-2,4a,5,6,7,7a-hexahydro-1H-[2]pyrindine-7-carboxylic acid::CHEMBL74395::SB-203207
SMILES: CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)CC(=O)N[C@]1([C@@H](O)C[C@@H]2[C@H]1CN(C)C=C2C(N)=O)C(O)=O
InChI Key: InChIKey=HDXFGBFIEQUETL-IVWWPFAYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoleucyl-tRNA synthetase (Staphylococcus aureus) | BDBM50093003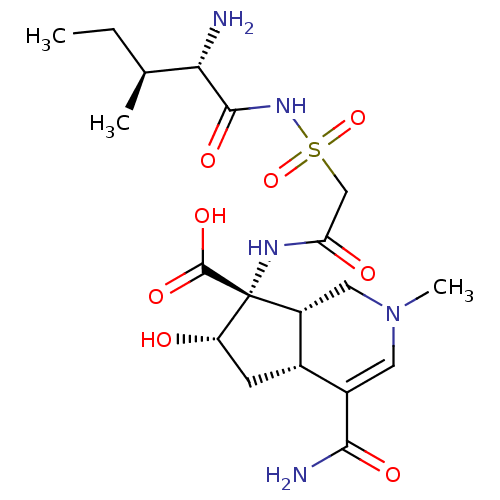 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-tRNA synthetase (Homo sapiens (Human)) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valyl-tRNA synthetase 2 (Homo sapiens (Human)) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||