

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50366387 STREPTOMYCIN
SMILES: [#6]-[#7]-[#6@H]-1-[#6@H](-[#8])-[#6@@H](-[#8])-[#6@H](-[#6]-[#8])-[#8]-[#6@H]-1-[#8]-[#6@H]1-[#6@H](-[#8]-[#6@@H]-2-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@@H](\[#7]=[#6](/[#7])-[#7])-[#6@H](-[#8])-[#6@H]-2\[#7]=[#6](/[#7])-[#7])-[#8]-[#6@@H](-[#6])[C@]1([#8])[#6]=O
InChI Key: InChIKey=UCSJYZPVAKXKNQ-ZCTIVXDHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50366387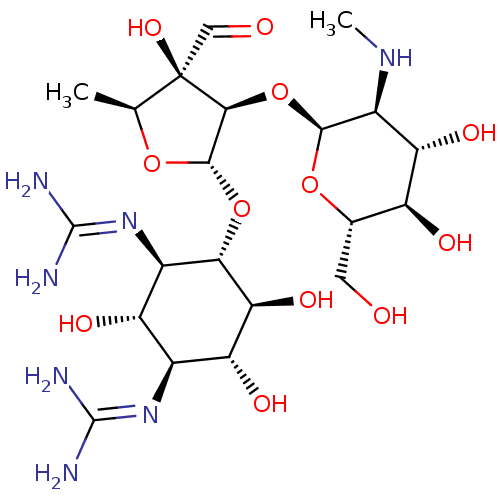 (STREPTOMYCIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of PAD4 measured by slope plot of Lineweaver-Burke analyses | Bioorg Med Chem 16: 739-45 (2008) Article DOI: 10.1016/j.bmc.2007.10.021 BindingDB Entry DOI: 10.7270/Q24F1RKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50366387 (STREPTOMYCIN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Ability to inhibit the binding of [125I]RT155 to Serotonin transporter in HEK cells | Hepatology 60: 1015-22 (2014) Article DOI: 10.1002/hep.27206 BindingDB Entry DOI: 10.7270/Q2TF00N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50366387 (STREPTOMYCIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Inhibition of PAD4 by ABPP-based assay | Bioorg Med Chem 16: 739-45 (2008) Article DOI: 10.1016/j.bmc.2007.10.021 BindingDB Entry DOI: 10.7270/Q24F1RKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||