

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@H]1CC[C@@]2(C)[C@@H](CCC=C2C)[C@]1(C)Cc1cc(O)ccc1O
InChI Key: InChIKey=JSPUCPNQXKTYRO-LWILDLIXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50242257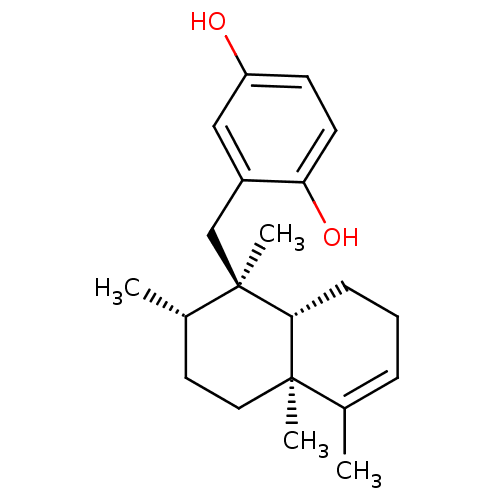 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ... | J Nat Prod 79: 1842-7 (2016) Article DOI: 10.1021/acs.jnatprod.6b00367 BindingDB Entry DOI: 10.7270/Q20V8FRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50242257 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for Cholesteryl ester transfer protein (CETP) inhibition by CETP-SPA assay | Bioorg Med Chem Lett 5: 605-610 (1995) Article DOI: 10.1016/0960-894X(95)00081-4 BindingDB Entry DOI: 10.7270/Q20R9PWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242257 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate incubated for 15 mins followed by substrate addition by Ellma... | Eur J Med Chem 122: 326-338 (2016) Article DOI: 10.1016/j.ejmech.2016.06.036 BindingDB Entry DOI: 10.7270/Q2QJ7K8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Chlorocebus aethiops) | BDBM50242257 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT2 expressed in CHO cells using [1-14C]oleic acid incubated for 6 hrs by TLC based method | Bioorg Med Chem Lett 29: 2283-2285 (2019) Article DOI: 10.1016/j.bmcl.2019.06.026 BindingDB Entry DOI: 10.7270/Q2NK3JC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Chlorocebus aethiops) | BDBM50242257 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of African green monkey SOAT1 expressed in CHO cells using [1-14C]oleic acid incubated for 6 hrs by TLC based method | Bioorg Med Chem Lett 29: 2283-2285 (2019) Article DOI: 10.1016/j.bmcl.2019.06.026 BindingDB Entry DOI: 10.7270/Q2NK3JC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Cricetulus griseus) | BDBM50242257 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | PDB MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of SOAT1 in CHO cell microsomal fraction using [1-14C]oleoyl-CoA as substrate measured after 5 mins by TLC based method | Bioorg Med Chem Lett 29: 2283-2285 (2019) Article DOI: 10.1016/j.bmcl.2019.06.026 BindingDB Entry DOI: 10.7270/Q2NK3JC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50242257 (2-((1R,2S,4aS)-1,2,4a,5-Tetramethyl-1,2,3,4,4a,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of COX2 | J Nat Prod 68: 985-91 (2005) Article DOI: 10.1021/np049655u BindingDB Entry DOI: 10.7270/Q27S7PN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||